Published online Dec 21, 2006. doi: 10.3748/wjg.v12.i47.7621
Revised: November 5, 2006
Accepted: November 13, 2006
Published online: December 21, 2006
AIM: To characterize the expression and dynamic changes of bone morphogenetic protein (BMP)-2 in hepatocytes in the regenerating liver in rats after partial hepatectomy (PH), and examine the effects of BMP-2 on proliferation of human Huh7 hepatoma cells.
METHODS: Fifty-four adult male Wistar rats were randomly divided into three groups: A normal control (NC) group, a partial hepatectomized (PH) group and a sham operated (SO) group. To study the effect of liver regeneration on BMP-2 expression, rats were sacrificed before and at different time points after PH or the sham intervention (6, 12, 24 and 48 h). For each time point, six rats were used in parallel. Expression and distribution of BMP-2 protein were determined in regenerating liver tissue by Western blot analysis and immunohistochemistry. Effects of BMP-2 on cell proliferation of human Huh7 hepatoma cell line were assessed using an MTT assay.
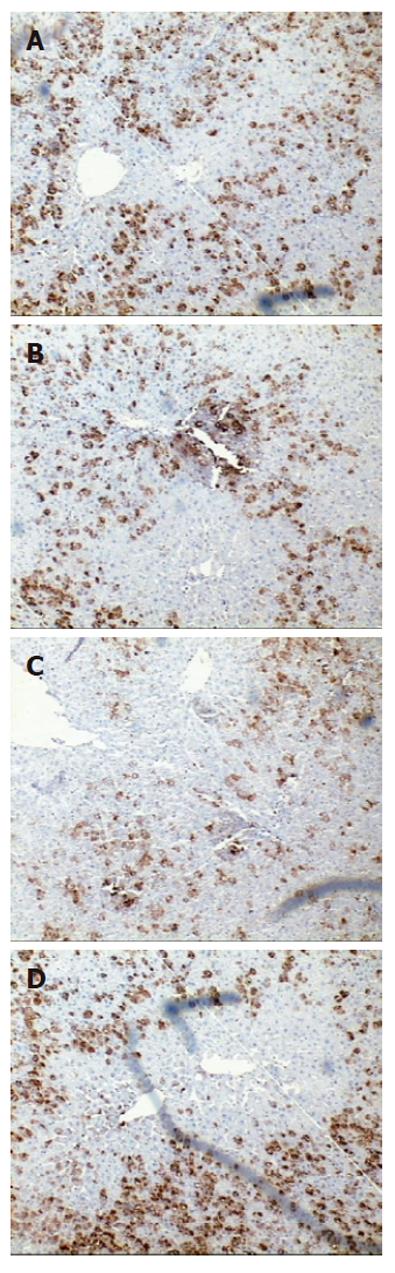
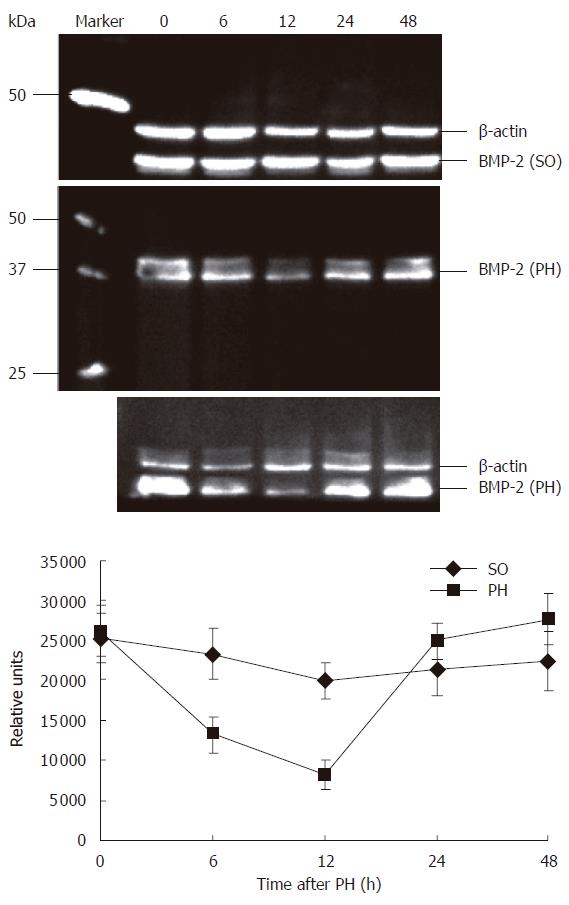
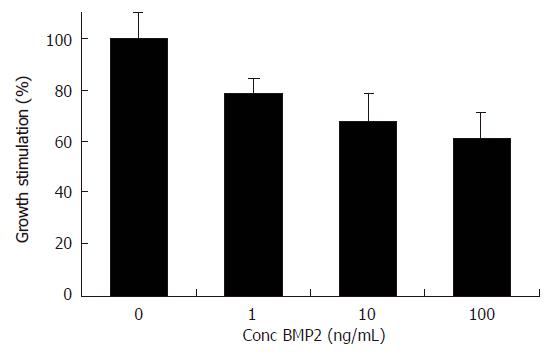
RESULTS: In the normal liver strong BMP-2 expression was observed around the central and portal veins. The expression of BMP-2 decreased rapidly as measured by both immunohistochemistry and Western blot analysis. This decrease was at a maximum of 3.22 fold after 12 h and returned to normal levels at 48 h after PH. No significant changes in BMP-2 immunoreactivity were observed in the SO group. BMP-2 inhibited serum induced Huh7 cell proliferation.
CONCLUSION: BMP-2 is expressed in normal adult rat liver and negatively regulates hepatocyte proliferation. The observed down regulation of BMP-2 following partial hepatectomy suggests that such down regulation may be necessary for hepatocyte proliferation.
- Citation: Xu CP, Ji WM, Brink GRVD, Peppelenbosch MP. Bone morphogenetic protein-2 is a negative regulator of hepatocyte proliferation downregulated in the regenerating liver. World J Gastroenterol 2006; 12(47): 7621-7625
- URL: https://www.wjgnet.com/1007-9327/full/v12/i47/7621.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i47.7621
Bone morphogenetic proteins (BMPs) were first identified in the 1960s[1]. BMPs are multi-functional growth factors that belong to the transforming growth factor beta (TGF-β) superfamily[2]. Mature BMPs are 30-38 kDa proteins that utilize BMP receptors and intracellular SMADs to transduce their signals to regulate cell proliferation, differentiation, morphogenesis and apoptosis. The role of BMPs in embryonic development and in postnatal and adult animals has been extensively studied in recent years. In addition to their well recognized role in bone physiology, BMPs are known to regulate the development and homeostasis of other organs including the liver[3]. Previous research showed that a receptor for BMP-9 is expressed in the HepG2 liver tumor cells. HepG2 cells bind BMP-9 and undergo a proliferative response[4]. Northern blotting analysis demonstrated the presence of BMP-6 in non-parenchymal liver cells and a role for BMP-6 in the regeneration of liver tissue was proposed[5]. BMP signaling plays a critical role in the regulation of liver development. BMP signaling from the septum transversum mesenchyme is necessary to induce liver genes in the endoderm and the morphogenetic growth of the hepatic endoderm into a liver bud[6,7]. Similar to TGF-β signaling, BMP signaling has been implicated in the development of hepatic fibrosis as BMPs have been shown to stimulate activation of hepatic stellate cells, which results in their transdifferentiation to an α-smooth muscle antigen positive myofibroblast-like phenotype[8,9].
Among BMPs, BMP-2 has gained more attention because it is the predominant form in natural bone morphogenetic protein extracts[10], and it is widely expressed during mouse development[11]. Researchers find that high affinity receptors for BMP-2 are present not only on osteoblastic cells but also on a large variety of non-hematopoietic cell types[12]. Preclinical and clinical studies have suggested that recombinant BMP-2 may have therapeutic potential in bone repair[13].
A hallmark of developmental biology is that similar pathways are involved in multiple different systems. It is clear that the role of BMP signaling is not restricted to the bone. The liver, just as the bone, has a remarkable regenerative potential, which involves tightly regulated molecular mechanisms that control hepatic proliferation, differentiation and morphogenesis after the loss of hepatic tissue[14]. However, the role of BMP signaling in the regenerating liver remains unclear. Here we focus on the expression and dynamic changes of BMP-2 in hepatocytes in regenerating liver of adult rats.
Fifty-four adult and healthy male Wistar rats weighing 180-220 g, obtained from the Animal Center of Shanxi Medical University, were employed in the present study. All rats received humane care during the study under a protocol that was in accordance with institutional guidelines for animal research and was approved by the Ethics and Research Committee of Shanxi Medical University. Experimental rats were randomly divided into three groups: Normal control (NC, n = 6) group, partial hepatectomized (PH, n = 24) group and sham operation (SO, n = 24) group. Rats were sacrificed at 6, 12, 24 and 48 h after partial hepatectomy or sham operation. For each time point indicated, six rats (n = 6) were used in parallel.
Rats were maintained on a 12/12 h light-dark cycle. The surgery was performed between 8 and 10 AM. Rats were fasted 12 h before surgery and anesthetized with pentobarbital sodium (30 mg/kg) intra-abdominally, then the abdominal skin was shaved and sterilized with an iodine solution. Two-thirds hepatectomy was performed as described by Higgins and Anderson. In the sham operated rats the liver was manipulated but not resected. Rats were anesthetized with pentobarbital sodium (20 mg/kg) intra-abdominally and killed at 6, 12, 24 or 48 h after partial hepatectomy or the sham procedure. The remnant livers were removed, parts of which were fixed 24 h in 10% buffered neutral formalin. The fixed livers were dehydrated through increasing concentrations of ethanol and in xylene and embedded in paraffin. Liver tissues embedded in paraffin were sectioned at 4 μm for immunohistochemistry. Part of the livers was snap frozen in liquid nitrogen for the preparation of protein lysates for Western blotting.
Immunohistochemistry was performed as described in detail below. Paraffin sections (4 μm) were dewaxed and dehydrated in graded alcohols. Endogenous peroxidase activity was quenched with 1.5% H2O2 in PBS for 30 min at room temperature. Antigen retrieval was performed by heating for 10 min at 95°C in 0.01 mol/L sodium citrate, and non-specific staining was blocked with TENG-T (10 mmol/L Tris, 5 mmol/L EDTA, 0.15 mol/L NaCl, 0.25% gelatin, 0.05% [vol/vol] Tween-20, pH 8.0) for 30 min at room temperature. Endogenous avidin binding activity due to biotin was overcome by successive 20 min incubations of the tissue sections in 0.1% avidin and 0.01% biotin (DAKO Biotin Blocking System). After a washing with PBS (3 × 5 min), BMP-2 primary antibody (mouse monoclonal BMP-2, MAB355, 1:500, R&D) was applied in PBS containing 1% bovine serum albumin and 0.1% Triton and incubated overnight at 4°C. The following day, for BMP-2 staining, sections were incubated with biotinylated goat-anti-mouse IgG (DAKO 1:200) for 60 min in PBS with 10% human serum, then washed in PBS (3 × 10 min) and incubated for 60 min with streptavidin-biotin-horse-radish peroxidase (DAKO) for 1 h, and washed again 3 × 5 min in PBS. And peroxidase activity was detected with DAB (Sigma), resulting in the formation of a brown reaction product. Finally, sections were briefly counterstained with hematoxylin, then dehydrated, cleared and mounted in neutral gum under cover slips. For controls, the primary and secondary antibodies were substituted and an appropriate IgG control (mouse IgG2b was applied at 1:50) was used to perform negative control staining. A known positive staining specimen (bone tissue of rat) was used as a positive control.
Rat liver tissue was homogenized in lysis buffer. Protein concentrations were measured using the Bradford method. Lysates were diluted as per 300 μL protein sample buffer was added with 600 μg extract in 2 × protein sample buffer and 30 μL of each sample of homogenates was loaded per lane on an SDS-PAGE gel. Equal protein loading was confirmed using β-actin (Santa Cruz) antibodies on the same blots after stripping off the old antibodies in stripping buffer. After protein separation the proteins were blotted onto a PVDF membrane (Millipore, Bedford, MA). The membranes were blocked with 2% protein (Nutricia, The Netherlands) in PBS supplemented with 0.1% Tween-20 for 1 h at room temperature. After a brief wash in washing buffer (0.2% protifar: 0.1% Tween-20), membranes were incubated overnight at 4°C with primary antibody (mouse monoclonal BMP-2, MAB 355, 1:1000, R&D systems) in 2% blocking buffer. The following day, membranes were washed three times for 5 min, and subsequently incubated with a secondary horseradish peroxidase (HRP)-conjugated antibody in wash buffer (0.2% low fat milk powder) at 1:1000 dilution. After enhanced chemoluminescence using Lumilight + substrate (Roche, Mannheim, Germany), antibody binding was visualized using a Lumi-Image-Pro Plus 5.0.
The Huh7 human hepatoma cell line[15] was obtained from the ATCC (American Type Culture Collection) and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM)(Gibco, Paisley, Scotland) with 4.5 g/L glucose and 1% L-glutamine. This was supplemented with penicillin (50 U/mL), streptomycin (50 μg/mL) and 10% FBS (Gibco). Cells were grown in monolayers in a humidified atmosphere containing 5% CO2. Eighty to ninety percent confluent monolayers of Huh7 cells were trypsinized and taken up in medium with 10% FBS. Cells at 5 × 104 per well were seeded in triplicate in flat-bottomed tissue culture of 24 well plates (Falcon) overnight in DMEM medium containing 10% FBS in the absence or presence of recombinant human BMP-2 (rhBMP-2 355-BM, R&D systems) at indicated concentrations for 72 h. MTT [3-(4,5-methylthiazol-2-yl)-2,5-dipheyl-tetrazolium bromide] reagent was added to all wells for 30 min. The medium was removed from the cells, and cells were lysed in acidic isopropanol and absorbance was measured at 550 nm with an enzyme-linked immunosorbent assay plate reader (Molecular Devices, Ther/vio max microplate reader).
Values are expressed as mean ± SE. Results were evaluated using analysis of variance and correlated by SPSS11.0 software. P < 0.05 was considered significant.
To determine the expression and localization of BMP-2 in the regenerating rat liver, immunostaining was carried out using a specific monoclonal anti-BMP-2 antibody. As evident from Figure 1, clear and strong BMP-2 immunoreactivity was present in the cytoplasm of hepatocytes around the central vein (CV) and portal triad (PT) in normal liver. In contrast, immunoreactivity of BMP-2 declined significantly at 12 h after PH. Thereafter the immunoreactivity of BMP-2 increased progressively and was back to normal levels at 48 h after PH. No significant change in BMP2 expression in SO group compared to normal controls was observed (data not shown).
To confirm the specificity of the observed changes in BMP-2 expression in the regenerating rat liver, levels of BMP-2 expression were determined by Western blot analysis. As shown in Figure 2, using the antibody a band at approximately 37 kDa was detected on the immunoblots, which corresponds to BMP-2 precursor protein. Quantification of BMP-2 expression showed a 3.22 fold reduction (P < 0.01) of BMP-2 protein expression compared to controls at 12 h after PH. Similar to the results obtained by immunohistochemistry, BMP-2 protein levels returned to control levels at 48 h after PH. There were no significant changes in BMP-2 expression in the SO group compared to normal controls.
Because BMP-2 expression was down regulated during liver regeneration, we next attempted to determine whether BMP-2 was capable of modulating Huh7 cell proliferation. To this end, Huh7 cells were treated with increasing concentrations of rhBMP-2. MTT assay demonstrated that proliferation of Huh7 cells was dose dependently inhibited by rhBMP-2. A maximal inhibition of 38.8% ± 0.4% (P < 0.01) was observed at 100 ng/mL (Figure 3).
Following partial hepatectomy, there is a rapid and highly orchestrated series of biochemical events that regulate hepatic regeneration. This is a complex process, which allows for a short period of rapid cellular proliferation but is subsequently followed by cell cycle arrest and cellular differentiation[14]. It has been shown that BMP signaling plays a critical role in hepatogenesis during endodermal patterning[6]. However, the role of BMPs in hepatic regeneration in the adult has not been studied.
The present study analyzed the expression of BMP-2 in normal rat liver. We studied the changes in BMP-2 expression during liver regeneration in the two-thirds hepatectomy model in rats. Immunohistochemical analysis showed that strong BMP-2 immunoreactivity was present in the cytoplasm of normal rat hepatocytes surrounding CV and PT. Both immunohistochemistry and Western blot analysis showed that BMP-2 declined significantly at 12 h after partial hepatectomy and returned to normal at 48 h. We showed that BMP-2 suppressed growth of Huh7 hepatoma cells in vitro, suggesting that BMP signaling negatively regulates hepatocyte proliferation. However, this in vitro observation needs further confirmation in vivo in a model of hepatocyte regeneration.
Our data remain descriptive but suggest that the role of signaling by BMP-2 may be distinct from that of TGF-β1, another TGF-β family member that signals through a different receptor complex. TGF-β1 is also an inhibitor of hepatocyte proliferation[16]; however, the normal liver expresses very low levels of TGF-β1. Levels of TGF-β1 expression increase rapidly after partial hepatectomy and peak at 12 h[17] exactly when the expression of BMP-2 is at its lowest. In mice with a liver specific deletion of TGF-β receptor type II, which is required for TGF-β signaling, increased hepatocyte proliferation and liver mass in response to partial hepatectomy was resulted[18], indicating that TGF-β signaling acts as a negative feedback loop in hepatic regeneration that keeps the mitogenic response in check. The dynamic expression of BMP-2 suggests a distinct role for this pathway. BMP-2 is readily detected in the normal liver and its expression rapidly declines after partial hepatectomy. This may suggest that BMP-2 signaling does not act in a negative feedback loop in hepatocyte regeneration but that instead, its down regulation may be necessary for the initiation of hepatocyte proliferation. Hepatocytes are the first to proliferate after partial hepatectomy. Hepatocyte proliferation started exactly in the area around the CV where BMP-2 expression was lost well before the onset of proliferation which peaked at 24 h[19].
In conclusion, both the localization and timing of expression of BMP-2 suggest that BMP-2 may be a negative regulator of hepatocyte proliferation that needs to be down regulated in order to allow the initiation of hepatocyte proliferation around the CV. Our data remain descriptive and interventional studies need to be performed to test this hypothesis.
The role of BMP signaling is not restricted to the bone. The liver, just as the bone, has remarkable regenerative potential which involves tightly regulated molecular mechanisms that control hepatic proliferation, differentiation and morphogenesis after the loss of hepatic tissue.
A hallmark of developmental biology is that similar pathways are involved in multiple different systems.
We aimed to summarize and emphasize the differences, from other related or similar articles so that readers may catch up the major points of the article easily.
This study examines the role of BMP-2 in hepatocyte proliferation following PH and in vitro in a human hepatoma cell line. BMP-2 has been shown to play an important role in bone development and may also be important in liver development. The authors proposed that this cytokine may act as a growth inhibitor during liver regeneration. The study is overall well designed and potentially important.
S- Editor Liu Y L- Editor Zhu LH E- Editor Liu WF
| 1. | Urist MR. Bone: formation by autoinduction. Science. 1965;150:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4079] [Cited by in RCA: 3691] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 2. | Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2955] [Cited by in RCA: 2824] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 3. | Sakou T. Bone morphogenetic proteins: from basic studies to clinical approaches. Bone. 1998;22:591-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 260] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 4. | Song JJ, Celeste AJ, Kong FM, Jirtle RL, Rosen V, Thies RS. Bone morphogenetic protein-9 binds to liver cells and stimulates proliferation. Endocrinology. 1995;136:4293-4297. [PubMed] |
| 5. | Knittel T, Fellmer P, Müller L, Ramadori G. Bone morphogenetic protein-6 is expressed in nonparenchymal liver cells and upregulated by transforming growth factor-beta 1. Exp Cell Res. 1997;232:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 469] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 7. | Zhang W, Yatskievych TA, Baker RK, Antin PB. Regulation of Hex gene expression and initial stages of avian hepatogenesis by Bmp and Fgf signaling. Dev Biol. 2004;268:312-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Shen H, Huang G, Hadi M, Choy P, Zhang M, Minuk GY, Chen Y, Gong Y. Transforming growth factor-beta1 downregulation of Smad1 gene expression in rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G539-G546. [PubMed] |
| 9. | Shen H, Huang GJ, Gong YW. Effect of transforming growth factor beta and bone morphogenetic proteins on rat hepatic stellate cell proliferation and trans-differentiation. World J Gastroenterol. 2003;9:784-787. [PubMed] |
| 10. | Wozney JM, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Relat Res. 1998;26-37. [PubMed] |
| 11. | Bächner D, Schröder D, Betat N, Ahrens M, Gross G. Apolipoprotein E (ApoE), a Bmp-2 (bone morphogenetic protein) upregulated gene in mesenchymal progenitors (C3H10T1/2), is highly expressed in murine embryonic development. Biofactors. 1999;9:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Iwasaki S, Tsuruoka N, Hattori A, Sato M, Tsujimoto M, Kohno M. Distribution and characterization of specific cellular binding proteins for bone morphogenetic protein-2. J Biol Chem. 1995;270:5476-5482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Azari K, Doll BA, Sfeir C, Mu Y, Hollinger JO. Therapeutic potential of bone morphogenetic proteins. Expert Opin Investig Drugs. 2001;10:1677-1686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1175] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 15. | Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982;42:3858-3863. [PubMed] |
| 16. | Carr BI, Hayashi I, Branum EL, Moses HL. Inhibition of DNA synthesis in rat hepatocytes by platelet-derived type beta transforming growth factor. Cancer Res. 1986;46:2330-2334. [PubMed] |
| 17. | Jirtle RL, Carr BI, Scott CD. Modulation of insulin-like growth factor-II/mannose 6-phosphate receptors and transforming growth factor-beta 1 during liver regeneration. J Biol Chem. 1991;266:22444-22450. [PubMed] |
| 18. | Romero-Gallo J, Sozmen EG, Chytil A, Russell WE, Whitehead R, Parks WT, Holdren MS, Her MF, Gautam S, Magnuson M. Inactivation of TGF-beta signaling in hepatocytes results in an increased proliferative response after partial hepatectomy. Oncogene. 2005;24:3028-3041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Black D, Lyman S, Heider TR, Behrns KE. Molecular and cellular features of hepatic regeneration. J Surg Res. 2004;117:306-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |