INTRODUCTION
Ulcerative colitis (UC) and Crohn’s disease (CD) are the major forms of idiopathic inflammatory bowel diseases (IBD) of the intestine. UC and CD are both debilitating chronic disorders that afflict millions of individuals throughout the world with symptoms which impair function and quality of life. Whereas UC is confined to the colon and the rectum, CD may affect any part of the gut from the mouth to the perianal[1-4]. A multitude of clinical manifestations represent the expressions of IBD. These include diarrhea, rectal bleeding, abdominal discomfort, fever, anemia, and weight loss[1-3]. Both UC and CD tend to run a remitting-relapsing course affected by diverse environmental factors[1,3-5].
Despite the recognition of a genetic background together with environmental factors, which at present are thought to translate into an inappropriate inflammatory response in patients with IBD[3,4,6], currently our understanding on the immunopathogenesis of IBD is inadequate. Hence, up to now drug therapy of IBD has been empirical rather than based on sound understanding of disease etiology. Accordingly, while drug therapy initially appears successful in the majority of patients, it comes at the cost of significant side effects[7,8]. Further, up to now, first line medications for exacerbation of IBD include 5-aminosalicylic acid (5-ASA) or sulphasalazine (SZ) in combination with a corticosteroid with consideration of azathioprine (or 6-mercaptopurine) and nutritional support for some patients[1,9-14]. Treatment failure in patients with severe disease has often been an indication for colectomy in up to 40% of steroid refractory patients[10,15] although in recent years, cyclosporin A (CysA) has been introduced for corticosteroid refractory UC[15,16]. Despite being moderately effective in this clinical setting in reducing colectomy rates, there remain serious concerns over long-term efficacy and toxicity of CysA[17].
Currently, the view is that IBD is perpetuated by inflammatory cytokines like tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-8 and others[18,19]. Based on this perception, in recent years, anti-cytokine antibodies, notably the anti-TNF antibody, infliximab have been developed for the treatment of IBD[13], and the seemingly success of infliximab in CD[20,21] is hoped to be realized in patients with UC as well[22,23]. However, the enthusiasm towards biologicals is currently dampened by concerns about their long-term efficacy and safety profiles[24-29]. Taking infliximab as one example (that has been through extensive clinical evaluations), following the initial and subsequent administrations, antibodies to this agent emerge which potentially can reduce its efficacy[29]. Regarding their side effects, the literature on biologic therapy carries headlines like “Tumor necrosis factor antagonist therapy and lymphoma development;”[28] “Serious bacterial infections in patients with rheumatoid arthritis under anti-TNF-α therapy;”[27] “Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk;”[26] “Adverse skin reactions to anti-TNF-α;”[24] “Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies;”[30] “Anorectal carcinoma after infliximab therapy in Crohn’s disease”[31]. There is no shortage of many more warnings.
In the face of the overwhelming evidence for the involvement of various cytokines in the immunopath-ogenesis of IBD and the fact that peripheral blood granulocytes and monocytes/macrophages (GMs) are major sources of these cytokines[32,33], GMs appear logical targets in the treatment of IBD. Indeed, histological examination of the mucosal tissue in biopsy specimens from patients with active IBD reveals a spectrum of pathologic manifestations among which presence of an abundance of neutrophils relates specifically to clinical disease activity and severity of the disease[1-3,34-36]. The circulating activated GMs are elevated with increased survival time in active IBD[35-44]. Paradoxically, corticosteroids[45] which are given to most patients with active IBD and inflammatory cytokines[46] increase neutrophil survival time. In this article, the author reviews the therapeutic application of selectively depleting peripheral blood GM by adsorption apheresis (GMA) in patients with IBD with a major focus on UC. The underlying rationale is that selective removal of these cells that are otherwise destined for migration to the intestine reduces the inflammatory intensity, which in turn allows healing to take place. The author also presents arguments why GMA should be likened to an effective and safe biologic therapy in IBD.
THE STRATEGY FOR SELECTIVE LEUKOCYTAPHERESIS
The Adacolumn which is featured in this editorial is an example of a medical device that can selectively deplete activated myeloid leukocytes from peripheral blood in the GMA therapy of patients with IBD[39,40-58]. In essence, the treatment involves an extracorporeal approach in which patients’ blood is passed through a column (Adacolumn) that is filled with specially designed cellulose acetate beads of 2 mm in diameter known as leukocytapheresis carriers. Pre and post column blood cell counts have shown that the carriers adsorb from the blood which passes through the column about 65% of granulocytes, 55% of monocytes and a very small fraction of lymphocytes[41]. These are the leukocytes that bear the so-called FcγR and complement receptors. They include GM, small subsets of CD19+B lymphocytes and CD56+NK (natural killer) cells[59-61]. One novel feature of this treatment is that it involves removing from the body the effector cells rather than administering drugs. It is therefore not expected to induce dependency or refractoriness and the treatment has not been associated with serious side effects in a significant number of patients[39,40,47-58].
TREATMENT OF PATIENTS WITH SEVERE STEROID REFRACTORY UC
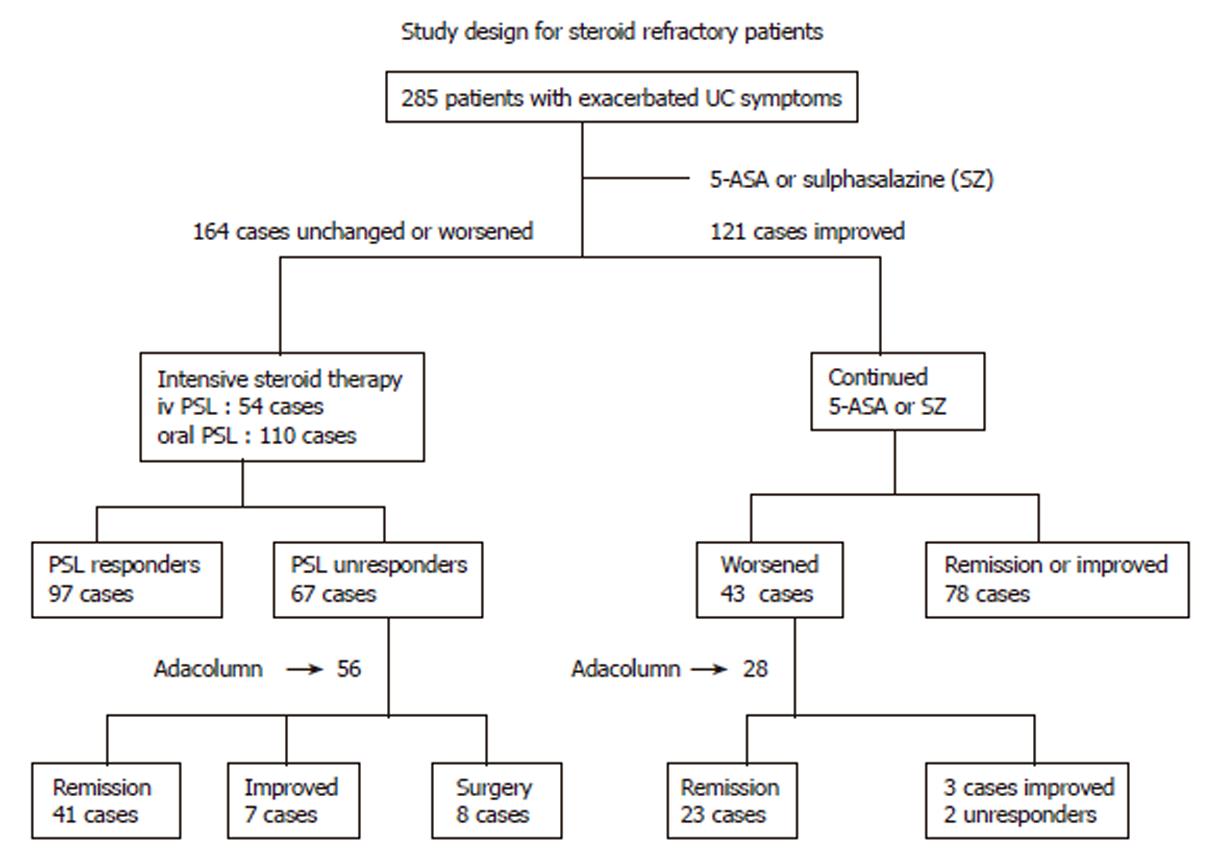
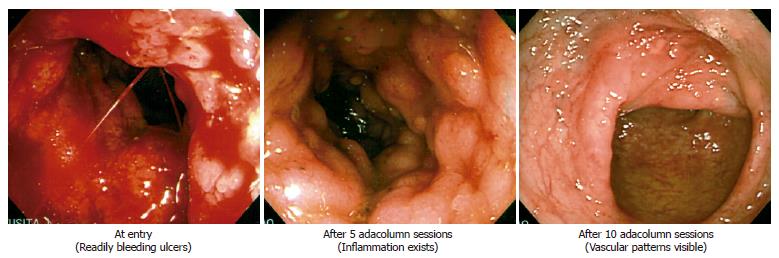
In our first major attempts (albeit without control groups)[39,40] we carefully selected patients with severe UC from a total of 285 patients with active UC who were first given salicylates as the first-line medication and those who did not improve (or worsened) were given intensive prednisolone (PSL) therapy and after a course of intensive PSL therapy, those who improved were not selected; 56 patients who could then be classified as steroid refractory were given GMA with the Adacolumn to deplete their peripheral blood GM. The study design is shown in Figure 1. The patients had a clinical activity index (CAI) of ≥ 12, a disease activity index (DAI) of ≥10[40,62,63] and were treated twice weekly for 2 to 3 consecutive weeks and then at one session per week for up to 11 GMA sessions. Assessments within one week after the last GMA session showed a response rate of 85% (Figures 1 and 2). No additional drug therapy was initiated while their ongoing PSL was tapered as symptoms improved. Figure 3 shows typical endoscopic improvements in steroid refractory patients. Pretreatment circulating neutrophil counts were very high, 9.3 × 109± 0.5 × 109/L, about 3 times the level seen in controls[40] and marked reductions were seen at wk 12 of treatment, 4.9 × 109± 0.4 × 109/L. Haemoglobin (Hb) at wk 12 relative to baseline increased by 25%, which may relate to the cessation of rectal bleeding following remission or improvements of clinical symptoms. Along with a fall in the patients’ CAI and DAI and peripheral blood leukocytes counts, there was a comparable fall in C-reactive protein[40]. A total of 11 non-severe side effects in 7 patients were observed during leukocyte reduction therapy[40]. These were 3 incidences of flushing, 6 incidences of dizziness/light headache, nausea in 1 and mild fever in 1. However, no patient discontinued GMA therapy due to these side effects, all of which lasted from a couple of minutes to 3 h. Further, there was no evidence of opportunistic infection in any patient during or after GMA therapy.
Figure 1 Study design and patient selection for selective leukocytapheresis with the Adacolumn (GMA) in patients with steroid refractory and steroid naïve ulcerative colitis.
iv prednisolone (PSL) indicates intravenous PSL (60 mg/d); oral PSL (40-60 mg/d). The dose of 5-ASA was 1.5-2.25 g/d while the dose of SZ was 2-3 g/d. During GMA course, PSL was tapered or discontinued in patients who improved. As shown, 56 steroid refractory and 28 steroid naïve patients were randomly selected for GMA, and the rest were treated according to Figure 5.
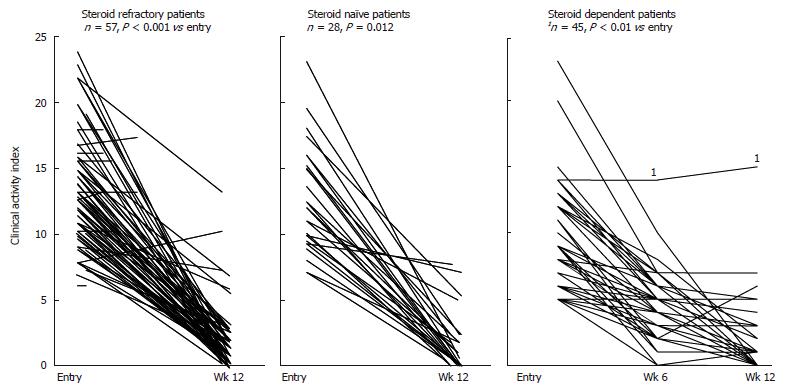
Figure 2 Fall of clinical activity index (CAI) during the course of Adacolumn GMA in patients with steroid refractory, steroid naïve and steroid dependent ulcerative colitis.
Figure 3 Typical endoscopic response in steroid refractory patients.
Modified from Hanai et al, Dig Dis Sci 2002; 47: 2349-2353 with kind permission of Springer Science and Business Media.
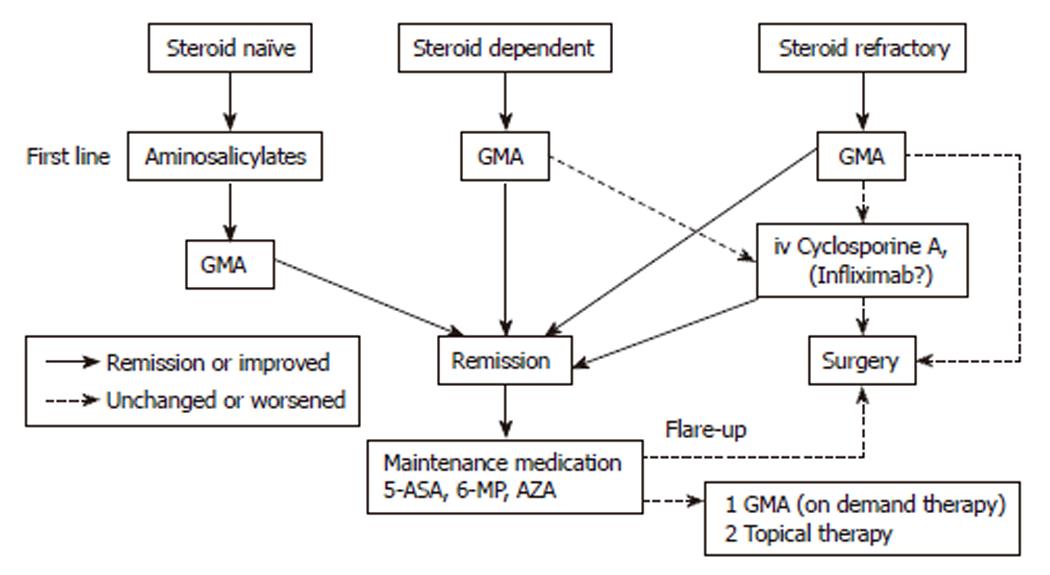
Figure 5 Treatment algorithm for ulcerative colitis without corticosteroids.
Based on this scheme, patients at any stage of their disease might benefit from GMA and avoid corticosteroids or other drug based medications. Intensive leukocytapheresis (2 or more GMA sessions per week in the first 3 wk) is recommended in severe or fulminating cases. GMA: Granulocyte and monocyte adsorption apheresis; 5-ASA: 5-aminosalicylic acid; 6-MP: 6-Mercaptopurine; AZA: Azathioprine.
The aforementioned refractory cases represent a sub-group of patients with severe UC who are at a significant risk of serious complications. Indeed treatment failure after 5-10 d of intensive corticosteroid therapy is often considered to be an indication for colectomy, CysA or TNF-α antibodies. Only 8 (14%) patients underwent colectomy. At 12 mo, 79% of patients had maintained their remission. In contrast, the relapse rate in patients who initially respond to CysA has been 60% to 80%[64], and unlike CysA, GMA with the Adacolumn has been without major side effects[39,40-58]. These initial response rates have subsequently been reproduced both in Japan and in Europe[47-58].
GMA AS A FIRST-LINE MEDICATION FOR STEROID NAÏVE PATIENTS
Together with our steroid refractory patients described above we had a subgroup of steroid naïve patients, all of whom achieved remission by GMA[40]. This has now increased to 28 patients (Figure 1). Most of these steroid naïve patients went into clinical remission by GMA and remained steroid naïve during the study and the 12-mo follow up period. Subsequently, Suzuki et al reported treating 20 steroid naïve patients with active UC by GMA[47,48]. The patients treated by Suzuki et al[47,48] had moderate to severe UC; mean CAI was 8.8. At entry, all patients were on 5-ASA (1.5 to 2.25 g/d). Each patient was to receive up to a maximum of 10 GMA sessions, at a frequency of 2 sessions/wk. Efficacy was assessed 1 wk after the last session. CAI fell to clinical remission levels (CAI ≤ 4) in the majority of patients after 6 sessions, and only 2 of the 20 patients required all 10 sessions. At post treatment, the mean CAI was 3, with a range from 0 to 12 and 17 of 20 patients (85%) were in clinical remission. The 3 non-responders had deep colonic ulcers at entry. There were significant changes in total peripheral white blood cell counts (× 109/L), 9.8 ± 1.0 vs 7.0 ± 0.6 at post treatment. In contrast, lymphocytes increased dramatically from a pretreatment level of 19% to nearly 30%, attributable to the increase in absolute lymphocyte count[47]. During GMA therapy, 2 incidences of transient mild headache were reported. In both cases, the headache receded within 3 h without medication.
GMA IN THE TREATMENT OF PATIENTS WITH STEROID DEPENDENT UC
Similarly, we used GMA to treat patients with corticosteroid dependent UC where GMA was used vs PSL[56]. A total of 261 consecutive patients who were initially evaluated were treated with a 5-ASA (1.5-2.25 g/d) or SZ (2-3 g/d). Patients who failed to respond were then treated with steroids and those patients who obtained remission, but relapsed during PSL tapering were given GMA or their steroid dose was increased. Both treatments were added to their ongoing conventional therapy. However, in both groups, PSL was to be tapered or discontinued in line with improvements of CAI. At wk 12, 83% in the GMA group and 65% in the PSL group were in remission (CAI ≤ 4)[56]. Further, in the GMA group, flushing was seen in 6 cases, nausea in 2 and mild fever in 2. This is in sharp contrast to 40 steroid side effects reported by Shimoyama et al[65] in a cohort of 52 patients who were given PSL. Typical remission rate in terms of CAI for GMA in steroid dependent patients is presented in Figure 2.
It is hard to overemphasize the clinical value of GMA in steroid dependent patients because patients in this group are exposed to steroids for most of their active disease lives and therefore, steroid side effects are often additional complications. Thus, one wary physician writes: How to do without steroids in inflammatory bowel disease[8] Quotations from this article include the followings: “I believe physicians have fallen into an incorrect pattern of using steroids without considering other therapeutic options;”“The toxicity associated with oral steroids occurs so frequently and is so severe that physicians should take another look at administering these agents;”“At a meeting of Crohn’s Disease and Ulcerative Colitis Foundation of America, a patient asked ‘Why physicians use steroids when they are so destructive to the individual ’” Therefore, Adacolumn GMA might be a safe and perhaps most effective alternative therapy for these patients.
GMA IN THE TREATMENT OF CD
The vast majority of clinical reports on GMA with the Adacolumn are in patients with UC. However, there is evidence to assume that GMA is effective in patients with CD as well. The first study in CD was reported by Matsui et al[54]. In that study, 7 patients with CD refractory to conventional medication including nutritional therapy, each received 5 GMA sessions. Five of seven patients achieved remission. In the study by Fukuda et al[55], 21 patients with refractory CD received 5 GMA sessions each. The efficacy rate was 52%. It is imperative to state that the patients Fukuda et al[55] included had received conventional medications including 2 wk of optimum nutritional therapy and only patients who remained with a high Crohn’s disease activity index (CDAI) were given GMA. Therefore, 52% remission rate in these refractory patients was very encouraging. Domenech et al[53] reported treating 12 steroid dependent patients with CD. The remission rate in that clinical setting was 70%. Finally, Muratov and colleagues[66] reported treating 7 patients with CD who were refractory or had relapsed despite medication. Six had received infliximab, but without success. Adacolumn GMA was performed at one session per week for 5 wk. Efficacy was assessed at wk 7 and 12 mo. The median value of CDAI decreased from 290 at wk 1 to 184 at wk 7 (P = 0.031). At the 12 mo follow-up, CDAI had decreased further to 128.5 (P = 0.0156).
WHAT COULD BE THE MOST APPROPRIATE DOSAGE OF GMA?
Since the publications of first papers on GMA in the treatment of patients with UC, the following questions have often popped up in the community of IBD physicians: What is the most effective number of GMA sessions for a patient with severe IBD What is the most appropriate frequency of GMA, one, two, three sessions per week or more How long should be the duration of one treatment session and treatment course The reality is that unlike drugs for which the dosage regimen has been defined, for a non-drug GMA, up to now treatment has been more or less arbitrary. In our centers, we have adopted a strategy of giving patients 2 treatment sessions per week in the first 2-3 wk and then 1 session per week up to 10 or 11 sessions[39,40,56]. We found that although patients with steroid naïve UC achieved remission or improved after 5 sessions, this was not seen in steroid refractory patients who responded better to 10 sessions[40]. Regarding duration of one GMA session, Kanke and colleagues[49], found that 90 min was significantly better than 60 min per GMA session. No data on the duration of one treatment course are available right now. However, the clinical response to Adacolumn GMA may not be immediately evident. For example, in patients with rheumatoid arthritis, there was a sustained increase in CD4+ T lymphocytes up to 12 wk following the last GMA session[41]. Similarly, there was a striking decrease in the expression of the chemokine receptor CXCR3 on leukocytes several weeks after the last GMA session[59,67]. Clearly it seems that much work has yet to be done before the optimum frequency and duration of treatment can be firmly established.
PATIENTS WHO ARE MOST LIKELY TO RESPOND TO GMA
Currently available data suggest that steroid-naive patients respond particularly well to this treatment[40,47]. Characteristically they respond faster with fewer GMA sessions and have a higher cumulative rate of remission[40,47]. As reviewed above, most steroid naïve patients in our centers achieved remission[40], while the remission rate in the cohort reported by Suzuki et al[47] was 85%. In one of the most thorough and retrospective studies by Suzuki and colleagues[58], the authors attempted to determine the responders to Adacolumn GMA. Their findings are summarized as follows. Seven days after the last GMA session, 20 of 28 patients had achieved clinical remission including all 8 patients who had their first UC episode. The mean duration of UC in the 8 first episode cases was 3.4 mo compared with 40.2 mo for all 28 patients and 65.4 mo for the 8 non-responders. The response to GMA seemed to be independent of basal CAI. The 8 non-responders were given conventional medication (CM) or CysA if the former failed. Two patients responded to CM, 3 to CysA and 3 underwent colectomy. The authors’ conclusions are as follows. First UC episode and short disease duration appear good predictors of response to GMA. Further, GMA might be an effective first line medication[57]. It would appear that the clinical response in patients with chronic continuous UC[40] and patients with deep colonic lesions[47] might be somewhat less satisfactory than in those experiencing their first UC.
GMA IN PATIENTS AT A HIGH RISK OF CLINICAL RELAPSE
Bjarnason and colleagues in London (Guy’s King’s and St Thomas’ Medical School) are currently evaluating the efficacy of GMA with the Adacolumn to suppress IBD relapse in patients at a high risk of experiencing one. The ongoing work was presented at the UEGW 2005 in Copenhagen[68]. The approach represents a fundamental change in the philosophy of treating IBD. Instead of treating active disease, asymptomatic patients are identified solely on the basis of a very high fecal calprotectin concentration, a neutrophil selective protein that provides quantitative measure of intestinal inflammatory activity[34-36]. The high calprotectin levels (over 250 μg/g) place them in a very high-risk group for clinical relapse[32]. This multi-center, prospective, randomized controlled study, assigned patients to Adacolumn, undergoing 5, once weekly, outpatient GMA sessions, or to unchanged treatment. Both patients with UC and CD were included. Follow up was monthly for 6 mo for a clinical relapse. In the Adacolumn group, 62% maintained their remission compared to 24% in the control group (P < 0.04, Pearson Chi squared test). Life table analysis demonstrated the mean survival in the Adacolumn group, 181 d vs 104 d in the control group (P = 0.016, Mantel Chi-squared test). This study represents a new approach to the treatment of IBD, namely targeting the inflammatory component of the disease at an asymptomatic stage. It seems likely that the 5 weekly sessions of GMA in such patients will have a significant effect and potentially avoid the morbidity associated with severe clinical relapses and the subsequent drug therapy in most patients.
EFFECTS OF GMA ON LEUKOCYTE-DERIVED INFLAMMATORY AND ANTI-INFLAMMATORY FACTORS
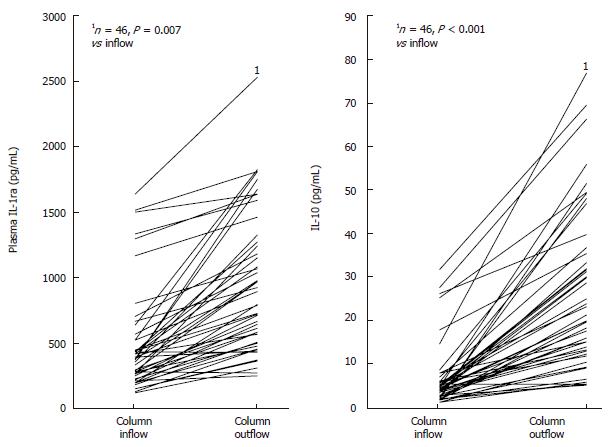
Although the primary target of GMA with the Adacolumn is to deplete activated peripheral blood leukocytes principally granulocytes and monocytes/macrophages, it has been difficult to explain why some patients continue to improve long after the treatment has been ceased. Also the low relapse rate during follow-up we have reported[40] can not be fully explained by our current understanding of neutrophil function or the effects of GMA on peripheral blood levels of leukocytes per se. Alternative mechanisms of action have therefore been sought. Adacolumn is filled with cellulose acetate beads to which leukocytes that bear the FcγR and complement receptors adhere[59-61]. The adsorbed leukocytes release an array of active substances both toxic and non-toxic, but anti-inflammatory as well[47,69-71]. Some of these like cytokines, complement fragment C3a and C5a are of short half-life and may not reach the patients’ circulation in fully active form. In view of this background, several investigators have carried out analysis on blood samples taken from the Adacolumn inflow and outflow (blood return line to patients) during GMA procedure. We[69,70] as well as Suzuki et al[47] found a significant increase in blood levels of soluble TNF-α receptors I, II. Soluble TNF receptors are believed to neutralize TNF without invoking TNF-like actions[72]. Further, several studies report a marked decrease in the capacity of peripheral blood leukocytes to generate inflammatory cytokines (TNF-α, IL-1β, IL-6 and IL-8) following Adacolumn GMA[39,41,59,65,71,73,74]. Also, GMA procedure appears to produce a similar effect on leukocyte trafficking receptors. Thus the expressions of both L selectin[59,74] and the chemokine receptor CXCR3[59,67] were dramatically reduced, while the expression of the leukocyte integrin, Mac-1 (CD11b/CD18) was up-regulated[41,59,73]. These observations indicate that the procedure has a suppressive effect on leukocyte extravasation. Similarly, in-vitro studies showed that incubation of human whole blood with the Adacolumn leukocytapheresis carriers for 60 min resulted in the generation of significant amounts of IL-1 receptor antagonist (IL-1ra) and hepatocyte growth factor (HGF)[75]. However, the authors did not detect significant amounts of TNF or IL-1. IL-1ra has an essential role in the control of inflammation in the mucosa[76,77] while HGF is known to promote mucosal epithelial cell regeneration, which is an essential step in ulcer healing[78]. These observations on cytokines and adhesion molecules are perhaps of more academic significance than therapeutic value, but still are related to the effects of the GMA on neutrophils. Figure 4 shows elevated IL-1ra and IL-10, another major anti-inflammatory cytokine[79] in the blood at the Adacolumn outflow.
Figure 4 Release of interle-ukin-1 receptor antagonist (IL-1ra) and IL-10 in the Adacolumn during Adacolumn GMA in patients with active ulcerative colitis.
Column outflow returns to the patients. Elevated IL-1ra and IL-10 (both anti-inflammatory) in the column outflow is potentially very significant (see text for comments on IL-1ra and IL-10).
There is evidence that GMA suppresses cytokine profiles within the mucosal tissue as well. Thus, Muratov et al[66] found a very marked decrease in tissue interferon (IFN)-γ positive T-cells in clinical responders (P = 0.027) after GMA. In parallel, significantly lower levels of IFN-γ producing lymphocytes were detected in peripheral blood. IFN-γ positive cells in pretreatment biopsies completely disappeared or decreased in post-treatment biopsies sampled 2 wk after the last GMA session in responders (P = 0.027) and appeared to predict the maintenance of long-term remission or response after 12 mo. In another study by Yamamoto and colleagues[80] , the authors found that at entry the mucosal concentrations of IL-1β, IL-1, IL-1ra, IL-6, IL-8 and TNF-α were significantly higher compared with healthy persons, while IL-1ra/IL-1β ratio was significantly lower. In patients who achieved clinical remission by GMA, but not in those without remission, the mucosal tissue concentrations of IL-1β, IL-1ra, IL-6, IL-8 and TNF-α significantly decreased, whereas the IL-1ra/IL-1β ratio significantly increased.
GMA INCREASES PERIPHERAL BLOOD LYMPHOCYTES
As stated above, the Adacolumn leukocytapheresis carriers selectively adsorb FcγR and complement receptors bearing leukocytes[59-61]. Indeed, Adacolumn not only spares the prevailing lymphocytes, it also induces an increase in de novo lymphocytes[41,47,81]. This is very intriguing and is in line with the original thinking for the design and development of the present Adacolumn, to tame the exuberant immune system in patients in whom an elevated peripheral neutrophils level was thought to promote disease progression[41]. One likely question could be “What is the merit of sparing lymphocytes ” The precise role of lymphocytes in the relapse of IBD is uncertain and evidence presented below indicates that indiscriminately removing peripheral lymphocytes even for a short period might in fact be pro-inflammatory in patients with IBD[71,82]. The majority of patients with active IBD have very low lymphocyte counts[47,81,83] and a low lymphocyte count has been associated with relapse of CD[83]. Hence depleting the already compromised lymphocytes potentially could impair adequate immune function. To our knowledge, there is no published data showing elevated peripheral lymphocytes in patients with active IBD. Further, in one of the best controlled studies on lymphocytapheresis in IBD reported by Lerebours and colleagues[84], the authors selectively depleted peripheral lymphocytes in patients with CD with the aim of suppressing clinical relapse. At the end of an 18-mo follow-up, the rate of relapse was 83% in the lymphocytapheresis group and 62% in the control group; the clinical outcome in the lymphocytapheresis group being 21% inferior to that of control. The increase in lymphocytes associated with Adacolumn GMA indicated above is primarily attributable to an increase in CD4+ T cells. Of these, the CD4+CD25+ T-cell subset suppresses intestinal inflammation through mechanisms that involve interleukin 10 and transforming growth factor beta[85,86].
CONCLUDING REMARKS
There have been significant recent advances in the medical therapy of IBD represented in part by the availability of biologicals which are developed to intercept the inflammatory cytokines or related inflammatory cells. However, the introduction of biologicals (albeit representing progress), has added new dimensions to the spectrum of treatment related adverse side effects like tuberculosis and lymphoma, to mention just two. Biologicals on which there rest great hope bear long term efficacy and safety concerns. Given that IBD is often associated with elevated and activated myeloid leukocytes which are major sources of inflammatory cytokines (the very agents that biologicals are expected to intercept), selective depletion of these leukocytes with the Adacolumn should represent a natural biologic therapy, and based on the available data, GMA seems to have an excellent safety profile. GMA in patients with steroid refractory UC (albeit mostly uncontrolled studies) has been associated with impressive clinical efficacy together with tapering or discontinuation of steroids, while in patients with steroid dependent or steroid naïve, GMA spares patients from exposure to steroids. Likewise, GMA at appropriate intervals in patients with IBD at a high risk of clinical relapse suppresses relapse thus sparing the patients from the morbidity associated with IBD relapse. Additionally, the procedure appears to reduce the number of patients being submitted to colectomy or exposure to potent immunosupressors like CysA. First UC episode, steroid naivety and short disease duration appear good predictors of response to GMA. This might be in line with the finding that corticosteroids support granulocytes. The author believes that GMA should be given as a first line medication. A treatment algorithm reflecting the author’s current opinion for the medical therapy of UC is presented in Figure 5.