Published online Dec 7, 2006. doi: 10.3748/wjg.v12.i45.7250
Revised: July 28, 2006
Accepted: September 4, 2006
Published online: December 7, 2006
Primary biliary cirrhosis (PBC) is an organ-specific autoimmune disease that predominantly affects women and is characterized by chronic, progressive destruction of small intrahepatic bile ducts with portal inflammation and ultimately fibrosis, leading to liver failure in the absence of treatment. Little is known about the etiology of PBC. PBC is characterized by anti-mitochondrial antibodies and destruction of intra-hepatic bile ducts. The serologic hallmark of PBC is the presence of auto-antibodies to mitochondria, especially to the E2 component of the pyruvate dehydrogenase complex (PDC). Current theories on the pathogenesis of PBC favor the hypothesis that the disease develops as a result of an inappropriate immune response following stimulation by an environmental or infectious agent. Some reports suggest that xenobiotics and viral infections may induce PBC. The pathogenetic mechanism is believed to be caused by a defect in immunologic tolerance, resulting in the activation and expansion of self-antigen specific T and B lymphocyte clones and the production of circulating autoantibodies in addition to a myriad of cytokines and other inflammatory mediators. This leads to ductulopenia and persistent cholestasis, by developing end-stage hepatic-cell failure. In this review are given our own and literary data about mechanisms of development of intrahepatic cholestasis and possible ways of its correction.
- Citation: Reshetnyak VI. Concept on the pathogenesis and treatment of primary biliary cirrhosis. World J Gastroenterol 2006; 12(45): 7250-7262
- URL: https://www.wjgnet.com/1007-9327/full/v12/i45/7250.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i45.7250
Primary biliary cirrhosis (PBC) is a chronic cholestatic, granulomatous, and destructive inflammatory lesion of the interlobular and septal bile ducts, which is likely to be caused by an autoimmune mechanism with a potential tendency to progress to cirrhosis[1,2].
Primary biliary cirrhosis is characterized by a T-cell-mediated destruction of bile duct epithelial cells that line the small intrahepatic bile ducts. This leads to ductulopenia and persistent cholestasis, by developing end-stage hepatic-cell failure.
The etiology of the disease is still unknown[3]. Many authors regard the disease as impaired immunoregulation with a loss of tolerance of histocompatibility antigen-enriched tissues. How and why the bile ducts are involved in this process remains unknown. Viruses[4,5], bacteria, xenobiotics, and human immunoregulatory defect may be possible PBC triggers that initiate the immunopathological cascade[6-8].
The development of PBC is preceded by a long asymptomatic period[9-11]. The wide use of computer-aided screening biochemical and immunological studies has significantly increased the detection of asymptomatic patients. In this period, there are generally no physical signs of PBC, at the same time anti-mitochondrial autoantibodies (AMA) are detectable in the serum of virtually all patients (95%)[12-15]. The fact that AMA are detectable many years before PBC manifests itself is indicative of their primary immunopathological role rather than a secondary phenomenon that occurs in the presence of cholestasis. The production of AMA is not an epiphenomenon, and an understanding of the mechanism of AMA induction will shed light on the etiology of PBC[3].
The activity of antibodies to antigens of various specificity (exogenous and autologous genesis) is associated with different classes of immunoglobulins. In PBC, there is an elevated concentration of the immunoglobulin M class (IgM)[16]. The increase in serum IgM is the result of chronic B-cell activation induced via the toll-like receptor (TLR) signaling pathway[17]. The activity of tissue, bacterial, and viral antibodies are assumed to be associated with the biological properties of IgM. Testing for AMA and IgM are the most useful laboratory procedure in the diagnosis of PBC[18].
Active searches are recently under way for autoantigens whose expression induces an immune response that results in destruction of the biliary epithelium. The antigens reacting with AMA are located on the internal mitochondrial membrane. The targets of activated T-lymphocytes are the dihydrolipoamide acetyltransferase components of the 2-oxoacid dehydrogenase, enzyme complexes that are important in oxidative energy metabolism. Pyruvate dehydrogenase complex (PDC) is the best known of these. Among the events demonstrated to induce an antibody response cross-reactive with PDC are exposures to bacterial PDC or retroviral proteins or xenobiotics or microchimerism[2]. Its dihydrolipoamide acetyltransferase component is referred to as PDC-E2[6]. PDC-Е1 and PDC-Е2 antigens are sensitive (98%) and specific (96%) for the diagnosis of PBC[19,20]. A major question in understanding the pathogenesis of PBC is why PBC patients lose their tolerance to antigens that are found in virtually every cell in the body. The identification of anti-PDC responses (present in over 95% of PBC patients) has given rise to important questions pertinent to our understanding of the pathogenesis of PBC. How and why does immune tolerance break down to as highly conserved and ubiquitously expressed self-antigen as PDC Why does the body stop recognizing individual components of the pyruvate dehydrogenase complex as proper proteins Why does breakdown in tolerance to an antigen present in all nucleated cells result in damage restricted to the intra-hepatic bile ducts How does the internal mitochondrial membrane antigen initiate the production of autoantibodies
Noteworthy is the assumption that infectious agents are involved in the etiopathogenesis of PBC. Based on the proposed role of microorganisms in the pathogenesis of the disease, Mao TK et al and Amano K et al hypothesize that patients with PBC possess a hyperresponsive innate immune system to pathogen-associated stimuli that may facilitate the loss of tolerance[21,22]. In PBC patients, AMA shows a cross-reaction with the subcellular components of gram-negative and gram-positive microorganisms[23].
Recent studies have suggested that the induction of PBC is multifactorial, in which the primary player involves the xenobiotics modification of mitochondrial proteins or exposure to xenobiotics-modified bacterial mitochondrial protein homologs, leading to breaking of tolerance to the human mitochondrial autoantigens and eventually liver pathology in genetic susceptible individuals[3,24,25]. A possible cause is molecular mimicry between microbial agents and self-antigens[26-28]. Infection with or exposure to a microorganism whose PDC-E2 bears a close homology with human PDC-E2 could act as an immunological trigger that initiates the development of PBC. It is suggested that the mutant forms of E. coli[26,29,30] and mycobacteria[31-33] trigger an immunopathological process in PBC. Anti-PDC-E2 antibodies cross-react specifically with mycobacterial hsp65[34]. Immunization of rabbits with E. coli rough (R) mutants gives rise to PBC-specific AMA. The PBC patients’ feces contain more or less counts of E. coli R-forms that specifically react with АМА[29]. Whether the intestinal R-forms are etiologically important for the development of PBC remains still unclear.
There has been recent evidence for the etiological role of Novosphingobium aromaticivorans in the development of PBC[35-37]. N. aromaticivorans is a gram negative strictly aerobic bacterium that is found worldwide in soil, water, and coastal plain sediments. Its PDC-E2-like proteins have a higher degree of homology with the immunodominant region of human PDC-E2 than any microorganism thus far studied (100-1000 times greater than that of Escherichia coli)[35,37]. N. aromaticivorans can metabolize xenobiotics that are similar to the chemical compounds that react with sera from PBC patients. Some of these xenobiotics are immunologically related to lipoic acid, the cofactor that is at the active center of PDC-E2. Thus, N. aromaticivorans can theoretically break down self-tolerance in two ways: by molecular mimicry due to subclinical infection and by the metabolism of xenobiotics that are present in the environment.
It was deduced that antibodies against N. aromaticivorans were found in 77 of 77 PBC patients from Milan, Italy, who had antibodies to PDC-E2 and that the titers to N. aromaticivorans proteins were similar to those to human PDC-E2[35,37]. Thirteen of 14 Icelandic PBC patients who were AMA positive reacted against at least one of the 2-oxoacid dehydrogenase-E2 complexes[36]. These observations provide additional evidence that exposure to N. aromaticivorans may trigger the development of PBC.
The epithelium of the bile ducts whose cell surface expresses antigens of the histocompatibility complex is considered to be the major target for AMA. The cross-interaction of AMA with the epitheliocytic antigens of bile cholangioles may damage the epithelium of ductules, by resulting in their obliteration. This leads to cholestasis in the minor canaliculi and bile capillaries with impaired bile excretion processes.
A morphologic study of liver biopsy specimens from asymptomatic patients with anti-mitochondrial autoantibodies detected during a screening makes it possible to diagnose one of the early stages of PBC. In stagesI-II PBC, the biopsy specimens show different phases of lesion of bile canaliculi. Early disorders develop in the interlobular ducts, 45-75 μm in diameter[38]. Dystrophy of ductal epitheliocytes should be considered to be the earliest disorder. Their cytoplasm becomes granular or homogeneous eosinophilic, swollen, and vacuolized, the nuclei get pycnotic. Later on, there is necrosis of a small segment of a canaliculus, but its outlines still remain and, finally, the wall is destroyed, that is a pattern of destructive cholangitis forms[39].
The generation of immune responsiveness to self-antigen can result in pathogenic autoimmune damage of the intrahepatic biliary epithelial cells mediated by both humoral and cellular immune responses[2,40]. In PBC osteopontin is an important immune molecule in portal tracts, and contributes to the recruitment of mononuclear cells into epithelioid granuloma and also participates in bile duct injury via B-cell differentiation and plasma cell expansion[41]. PBC is characterized by chronic destructive cholangitis with a Th1-predominant cytokine milieu[42-44]. CD8+ and CD69+ T cells were predominant in inflammatory infiltrates around damaged cholangiocytes; β2-microglobulin conformational epitope and intercellular adhesion molecule-1 expression were enhanced in bile ducts and hepatocytes[45,46]. Toll-like receptor-3 and type I interferon signaling pathways are active in both the portal tract and liver parenchyma of early-stage PBC, and form the basis for hypothesis that these signaling pathways are involved in the pathophysiology of PBC[47]. Oxidative stress- and nitric oxide-mediated cellular senescence may be involved in bile duct lesions, which are followed by progressive bile duct loss in primary biliary cirrhosis[48-51].
The canals of Hering (CoH) are destroyed in PBC in concert with the destruction of small bile ducts. This destruction appears to be an early event, because CoH numbers are lowest around stage 0 portal tracts, which still contain normal bile ducts[52]. The canals of Hering (CoH), converging from the hepatic lobule onto the portal tract, connect bile canaliculi to the interlobular bile ducts, and represent the most proximal portion of the bile drainage pathway with a cholangiocyte lining. De novo expression of intercellular adhesion molecule-1 (ICAM-1) both on mature cholangiocytes in CoH and epithelial cells in bile ductules in PBC implies that lymphocyte-induced destruction through adhesion by ICAM-1 and binding of lymphocyte function-associated antigen-1 expressing activated lymphocytes takes place not only in the bile ductules, but also in the CoH[53,54].
Thus, autoimmune pathological processes gradually leading to ductulopenia develop in asymptomatic PBC[55-57]. The increased number of desolating bile ductules gradually result in impairment of bile excretion and in deficient entry of bile acids into the duodenum. In response to this, the hepatocyte increases the synthesis of bile acids. But ductulopenia does not diminish and bile secretion fails to restore. The reduction in the intestinal level of bile acids through the feedback system induces a compensatory hepatocytic increase in the biosynthesis of bile acids and cholesterol, the major substrate for their biosynthesis. The progressively increased bile acid biosynthesis occurring with the participation of cytochrome P450 of the smooth endoplasmic reticulum and mitochondria leads, on the one hand, to hypertrophy of the endoplasmic reticulum and then to its hyperplasia and vacuolization, and, on the other, to the biosynthetic involvement of primary bile acids, other than specific P450-dependent monooxygenase and non-specific P450-dependent monooxygenase[58] that can oxidize cholesterol by the peroxide mechanism[59] to “atypical” metabolites. The latter may be further oxidized to “nonphysiological”, “atypical” bile acids in the mitochondria. Moreover, the hepatocytic concentration of bile acids gradually elevates and their entry into the intestinal lumen remains insufficient, giving rise to a closed vicious circle that results in the accumulation of bile acids in the liver cell. Due to the elevated hepatocytic level of bile acids, their reabsorption from the portal venous bed decreases, leading to the entry and progressive accumulation of bile acids in systemic circulation. This triggers the development of intrahepatic cholestasis that shows up as immunological, morphological, and biochemical signs when the disease is asymptomatic.
To inactivate the detergent effect of excess bile acids on the cell membrane apparatus, their sulfation and glucuronidization occur and the hepatocytic biosynthesis of cholesterol and phospholipids that are able to generate micellar-lamellar structures wherein bile acids are inserted diminishes. The biosynthesis of phospholipids occurs with the use of orthophosphate formed by hydrolysis of organic phosphorus compounds under the action of phosphatases: alkaline phosphatase (AP), and 5'-nucleotidase. The activity of these enzymes increases in patients with PBC just in the asymptomatic stage of the disease[16,60,61]. The increased activity of AP and 5'-nucleotidase is presumed to be due to their accelerated biosynthesis in the hepatocytes[62-64]. To enhance the synthesis of these enzymes, it is necessary to increase delivery of amino acids to the cells with the participation of γ-glutamyltransferase (GTP). A.S. Loginov[60] has noted that the change in blood γ-glutamyltransferase activity in patients with PBC outstrips the enhancement of the activities of AP and 5'-nucleotidase.
It is theorized that AP and 5'-nucleotidase give rise to orthophosphate where the latter is required[65]. Based on this theory, the enhanced activity of AP and 5'-nucleotidase in PBC is indicative of the higher hepatocytic need for orthophosphate.
High-resolution 31Р NMR spectroscopy has established the level of phosphate-containing compounds in the native bile of healthy individuals and PBC patients. There are lower levels of lecithin and orthophosphate in the patients’ hepatic bile portion (Table 1).
| Parameters | Values | |||
| Controls (mean1± SE) | Patients with PBC (mean2± SE) | mean1/ mean2 | ||
| Bile acids (g/L) | Bile | 3.9 ± 0.8 | 0.65 ± 0.02 | 6.0 |
| Blood | 0.012 ± 0.008 | 0.054 ± 0.008 | -0.2 | |
| Cholesterol (μmol/L) | Bile | 0.91 ± 0.06 | 0.38 ± 0.08 | 2.4 |
| Blood | 4.2 ± 0.6 | 11.8 ± 1.6 | -0.4 | |
| Lecithina | Bile | 2.1 ± 0.3 | 0.5 ± 0.1 | -4.6 |
| Orthophosphatea | Bile | 1.2 ± 0.3 | 0.30 ± 0.07 | 4.0 |
The data given in the table show the average statistical integral intensity of the corresponding 31Р NMR spectral signals in conditional units.
The change in the concentration of one of the native bile components-phosphatidylcholine (lecithin) suggests its altered acid-dependent (dependent on the secretion of bile acids) secretion by hepatocytes[66]. Biochemical studies have revealed that hepatic bile portions from PBC patients contain lower levels of not only lecithin, but also bile acids and cholesterol (Table 1). These findings suggest that hepatocytic bile secretion of the study components is decreased and that the relationship is retained between the secretion of bile lipid components (lecithin and cholesterol) and bile acids. At the same time there is an irregular reduction of bile acids and lipid components in the hepatic bile portion from patients with PBC. Thus, the levels of bile acids, lecithin, and cholesterol are decreased by 6, almost 5, and only 2.4 times, respectively, which is indicative of higher bile lithogenicity in these patients (Table 1).
The decreased quantity of orthophosphate in the hepatic bile portion from PBC patients points to its hepatocytic secretion in bile excretion and its simultaneous reduction in its liver cell content since the secretion of orthophosphate, as other inorganic ions, is passively effected by the concentration gradient and does not depend on that of bile acids. The reduced hepatocytic concentration of orthophosphate (despite the enhanced activities of AP and 5'-nucleotidase) indicates the intensive uptake of a phosphorus group in the metabolic processes taking part in the liver cell in PBC.
Biosynthesis of phospholipids is one of the possible ways of using orthophosphate[67]. The hepatic biopsy specimens of PBC show a 1.5-fold increase in the total amount of phospholipids[68]. This increase occurs, by elevating the content of lecithin in the cell membranes. Moreover, the same membranes contain the lower levels of lysophosphatidylcholine, sphyngomyelin, phosphatidylserine, phosphatidylinositol, and phosphatidyl-ethanolamine[68]. There is a 2-fold decrease in the ratio of free cholesterol to phospholipids in the cell membranes. Major alterations in protein status and lipid composition occur in the erythrocyte membrane of patients with PBC[69].
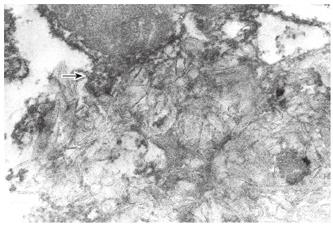
Along with this, segments of membrane cholestero-lization and those of free cholesterol deposition in the cytoplasm (Figure 1) and Disse’s space are detectable by electron histochemical studies of liver biopsy specimens from PBC patients, by using digitonin. All cholesterol-digitonin complexes show a more or less significant osmiophilia, which points to the presence of phospholipids and proteins in these complexes.
Yokomori H et al demonstrate the increased expression of caveolins in proliferating bile ductules in PBC, which may be related to the homeostasis of cholesterol transport in regenerating bile ductules in PBC liver[70]. Caveolins are cholesterol-binding proteins involved in the regulation of several intracellular processes including cholesterol transport.
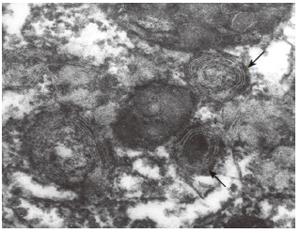
Electron microscopic studies of liver biopsy specimens from patients with PBC, including those with its early stage, have revealed ultrastructural alterations: hypertrophy and vacuolization of the smooth and rough endoplasmic reticulum, formation of lipid drops and myelin-like structures (Figure 2); the mitochondrial shape and structural changes characterized by the reduced number of internal membrane cristae, the appearance of external membrane "pseudopodia" (Figure 1), as well as local discrepancies of hepatocytes due to their intercellular space.
In the PBC biopsies, the enzymatic activity is increased in the bile canaliculi and is also present in the lateral membranes of the hepatocyte. Transmission electron microscopy of the lateral surface of the hepatocyte in normal livers showed a smooth surface without microvilli but in PBC livers a large number of microvilli were seen in the lateral membranes[71]. The localization of the enzymatic reaction, microvilli and Golgi apparatus at the PBC hepatocyte lateral membranes may represent a compensatory mechanism for derivation of bile flow and other components from the hepatocyte to the intercellular space.
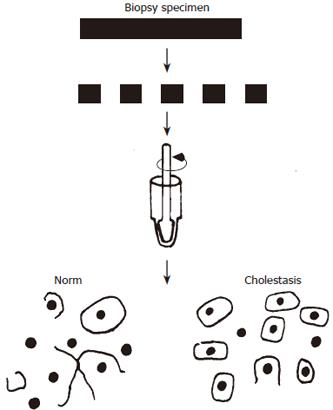
The results of electron microscopic studies of intercellular contact impairments in cholestasis agree well with the estimates of cell adhesion, by using the quantitative method developed by the authors to evaluate adhesive interactions in hepatocytes[72]. The essence of the method is as follows: tight junction (TJ) ensures a mechanical strength of interhepatocytic interactions and when liver tissue disperses with strong intercellular contacts, the plasma membrane breaks and the cell nuclei release. Decreased intercellular contacts lead to that in mechanical dispersion of liver tissue, the hepatocytes move away from each other, without rupturing the plasma membrane. A great number of single cells and much fewer cell nuclei are detectable (Figure 3).
After homogenization of a liver tissue biopsy specimen, single hepatocytes and free cell nuclei are detectable by light microscopy. Then the dissociation coefficient (Cd) is the ratio of the number of single cells (Nc) to the amount of single cells and the cell nuclei (Nn) isolated after liver tissue dispersion is calculated by the formula: Cd = Nc/(Nc + Nn).
A study of liver biopsy specimens indicated a statistically significant increase in Cd in patients with PBC as compared with those with chronic liver diseases, which were similar (Table 2). There is a 1.5-2-fold decrease in the size of hepatocytes. In PBC, the increase in Cd is associated with decreased intercellular contacts, particularly at the site of tight junction, probably due to cholestasis and its related elevated pressure in the bile capillaries. Substantial alteration in the TJ protein occurs predominantly in bile ducts and in hepatocytes in PBC, suggesting increased paracellular permeability along different paracellular routes for bile regurgitation in these chronic cholestatic liver diseases[73]. Melero S et al provide functional evidence that PBC cholangiocytes exhibit a widespread failure in the regulation of carriers involved in transepithelial H+/HCO3- transport, thus, providing a molecular basis for the impaired bicarbonate secretion in this cholestatic disease[74].
| Liver lesion | Cd (mean ± SD) | Cd ranges |
| PBC (n = 11) | 0.61 ± 0.03b | 0.41 ± 0.84 |
| CH (n = 20) | 0.14 ± 0.04 | 0.02-0.24 |
| LC (n = 3) | 0.18 ± 0.03 | 0.14-0.24 |
| UH (n = 8) | 0.11 ± 0.02 | 0.00-0.16 |
| FH (n = 11) | 0.07 ± 0.02 | 0.00-0.14 |
| Controls (n = 16) | 0.09 ± 0.02 | 0.00-0.18 |
There is no direct evidence for elevated hepatocytic levels of bile acids since methods for their determination within the liver cell are lacking. However, many indirect experimental data lead to the conclusion that liver cell lesion observed in chronic cholestasis is at least partially caused by the hepatocytic accumulation of excessive cellular toxic bile acids[75-77].
Osmiophilic myelin-like and cholesterol-digitonin structures detectable in the liver biopsy specimens by electron microscopic study appear to be intracellular located complexes of cholesterol, phospholipids, and bile acids.
The blood of patients with early-stage PBC contains higher levels of phospholipids, cholesterol[78,79], and bile acids[80-82] (Table 1). The change in the content of cholesterol, phospholipids, and bile acids in the blood of patients with PBC is associated with their increased formation in the liver and regurgitation into blood flow. Down-regulation of basolateral uptake systems and maintenance/up-regulation of canalicular and basolateral efflux pumps may represent adaptive mechanisms limiting the accumulation of toxic biliary constituents[83]. The elevated levels of phospholipids and cholesterol in the serum of patients with PBC seem to be determined by the necessity of neutralizing the detergent effect of excess bile acids entering the systemic circulation. Cholesterol and phospholipids are able to bind bile acids, by inactivating their solubilizing effect. This gives rise to the so-called micellar-lamellar structures that are water soluble.
Protracted and steady-state elevations in the concentration of cholesterol in the blood of patients with PBC give rise to xanthelasmas. There is a relationship between the development of cutaneous xanthelasmas and the elevated serum levels of cholesterol. According to the data obtained by HG Kunkel & EH Ahrens[84], cutaneous xanthelasmas appear when the blood concentration of cholesterol is more than 450 mg/dL. Moreover, this cholesterol level should persist for at least 3 mo. Hyperlipidemia in PBC does not seem to be associated with an increased risk of atherogenesis[85].
The normal (or slightly enhanced) activity of aminotransferases for several years[86] suggests the preserved integrity and normal permeability of the cytoplasmic membrane of hepatocytes in most patients with PBC.
Skin hyperpigmentation is due to the excessive biosynthesis of melanin, as shown by S Sherlock[87]. The initial reaction of melanin biosynthesis is catalyzed by tyrosinase, the copper-containing enzyme. The liver is known to play an important role in the metabolism and regulation of copper homeostasis due to the formation of hepatocytic complexes of protein and copper and its bile excretion[39]. In normalcy, about 80% of the dietary copper is excreted into bile and eliminated with feces. Disturbance bile formation processes in PBC lead to the accumulation of copper in the body. Excess serum copper levels in patients with PBC may cause increases in the activity of tyrosinase and in the biosynthesis of melatonins that deposit in the skin, inducing hyperpigmentation. Simultaneous deposition of copper in the skin imparts a bronzed tint. Since copper is in ceruplasmin-bound state, its elevated content exerts no toxic effect on the body, as in the Wilson-Konovalov disease.
Further progression of intrahepatic cholestasis results in the occurrence other of clinical signs of the disease.
Local or diffuse, moderate or severe skin itching is the earliest and permanent, and, occasionally for several months, sole symptom of the disease[88]. Epidermal deposition of bile acids resulting from cholestasis with PBC patients is a cause of skin itch[89].
At the same time, all fractions of conjugated bile acids increase in the blood[90] while in the skin 50%-85% of bile acids are not conjugated with glycine or taurine[91] and less than 20% of bile acids are in the form of sulfoesters[92]. Sulfation and glucuronization of bile acids diminish their toxic properties and increase their urinary and fecal excretion. The processes of acid bile sulfation and glucuronization, which occur in PBC, seem to be a response to the toxic effect of excess bile acids and to aim at enhancing their elimination through the skin, kidney, and intestine and at lowering their detergent and irritant effect on cells, tissues, and organs. On entering the skin, bile acids induce skin itch. The latter’s intensity depends on the ratio of sulfated (glucuronized) and nonsulfated (nonglucuronized) bile acids accumulating in the skin.
The sera of patients with PBC show a higher ratio of trihydroxy-/dihydroxycholanic acids and a lower glycine/taurine coefficient[90]. Garcia-Marin JJ et al[93] have indicated that taurine salts of bile acids stimulate the production of micelles with cholesterol and lecithin, promoting the inactivation of cholanic acids. Greim et al[94,95] have shown that cholic (trihydroxy-) bile acid has smaller detergent properties than dihydroxy- (deoxycholic, chenodeoxycholic) bile acids. The higher ratio of trihydroxy-/dihydroxy- bile acids in PBC as a compensatory detoxifying mechanism becomes apparent.
"Atypical", "nonphysiological" bile acids appear in the blood and urine of patients with PBC[96]. The appearance of "atypical" bile acids suggests that bile acid biosynthesis accomplished by the participation of 7-α and 12-α hydroxylases is impaired[80]. This may be due to the involvement into the process of biosynthesis of primary bile acids, apart from specific and nonspecific P450-dependent monooxygenases[58].
"Atypical" bile acids have a more potent irritant effect on the nerve receptors located in the skin. The intensity of skin itch also depends on the amount of "nonphysiolo-gical" ("atypical") bile acids in the skin of patients with PBC.
PBC affects mainly middle-aged, able-bodied females[97]. What is associated with is still unknown so far. Alterations in the content of sex hormones referring to as steroid ones are one of the possible causes of PBC in females[98]. There may be a change in the number or sensitivity of sex hormone receptors. The low expression of estrogen receptors-alpha in PBC and their disappearance in the advanced histological stages suggests that an estrogenic deficiency could favor the evolution of this disease toward ductopenia[99].
A study of steroids hormones in the plasma of patients with PBC has demonstrated a statistically significant reduction in the levels of progesterone and cortisol[100]. The found changes seem to be associated with the delayed biosynthesis of steroid hormones in late-stage PBC. This may be due to the competitive inhibitions of monooxygenases (by enhancing the biosynthesis of bile acids from cholesterol) that participate in the biosynthesis of progesterone and cortisol. Changes in sex hormone profile are secondary to hepatic dysfunction in PBC[98]. Lower cortisol concentrations in the blood of patients with PBC may cause a change in the fluidity of membranes[101,102], including hepatocytic cytoplasmic membranes. The changed fluidity of hepatocytic membranes leads to impaired bile excretion through the canalicular portion of a hepatocytic cytoplasmic membrane, which aggravates and enhances intrahepatic cholestasis.
Deficient entry of bile acids into the intestinal lumen substantially diminishes the absorption of fats and fat-soluble vitamins (А, D, and K)[103]. Along with insufficient vitamin D absorption, there is a lower formation of 1,25-dihydroxyvitamin D3 on cytochrome Р450 (competitive inhibition of monooxygenases due to the enhanced biosynthesis of bile acids). This all brings about the inadequate calcium ion absorption in the small bowel and impaired phosphorus-calcium metabolism. Impaired hepatocytic calcium metabolism may affect the contractility of intracellular microtubules and microfilaments, which may cause a decrease in the contractility of bile capillaries and enhance cholestasis[77].
Impaired phosphorus-calcium metabolism gradually results in osteodystrophy[104]. Signs of osteoporosis are most commonly detected in patients with end-stage PBC by bone X-ray studies[105,106] and morphological studies of bone biopsy specimens[87,107,108].
The pathogenesis of osteoporosis in cholestatic lesions of the liver appears to be multifactorial[109] and involves impairments of vitamin D3 absorption and metabolism[85], decreased intestinal calcium ion absorption[108,110], genetic predisposition[111], and impact of corticosteroid therapy[112].
Recent data suggest that serum leptin is associated with bone mineral density (BMD). F Szalay et al found a lower serum leptin level and a higher soluble leptin receptor in patients with PBC, which could not be explained by the difference in body mass index. As leptin was associated with BMD, it may be hypothesized that leptin is involved in the complex regulation of bone metabolism in PBC[113]. There was a clear increase in serum leptin levels according to the histological stage of PBC[114].
Glucocorticosteroids (GCSs) diminish intestinal calcium absorption, by reducing the production of 1, 25(OH)2-D3, increasing urinary calcium excretion, and depressing canalicular reabsorption. As a result, there is a compensatory increase in the production of parathyroid hormone (PTH) and bone resorption. In addition, GCSs directly increase PTH release and enhance its sensitivity. Furthermore, GCSs inhibit bone formation indirectly, by suppressing the synthesis of testosterone in gonads and by decreasing the production of growth hormone, insulin-like growth factor, and accordingly type 1 collagen, as well as directly by suppressing the function of osteoblasts[115,116]. Impaired fat absorption brings about steatorrhea - a daily fecal fat loss of more than 7 gram.
The altered composition of bile acids secreted into bile in patients with PBC leads to the impaired ratio of cholesterol, bile acids, and lecithin (in favor of cholesterol), which changes bile viscosity, and increases the bile lithogenicity index. This enhances evolving cholestasis in the bile capillaries and cholangioles. Blood bile reflux occurs. Another clinical sign of PBC, such as jaundice, develops. The development of jaundice is favored by attenuated interhepatocytic interactions and membrane fluidity changes leading to the impaired transport of bilirubin and copper through the canalicular portion of the hepatocytic cytoplasmic membrane. There is accumulation of copper in the body, activation of the copper-containing enzyme tyrosinase, an increase in the synthesis of melanins and in their skin deposition. Skin hyperpigmentation develops.
The serum concentration of bile acids increases and skin itch occurs in patients with PBC long before hyperbilirubinemia and jaundice develop in them. In end-stage PBC, an increase in the level of bilirubin occurs mainly due to its conjugated fraction. These data suggest that the development of hyperbilirubinemia in PBC is most likely to be associated with bile reflux from the bile capillary lumen or hepatocyte into blood and due to its impaired secretion through the apical portion of the hepatocytic cytoplasmic membrane. The altered fluidity of the liver cell membrane in PBC may promote impaired bilirubin transport and enhanced hyperbilirubinemia. Down-regulation of uptake transporters may contribute to the impaired hepatobiliary elimination in advanced PBC, and partially altered localization of MRP2 may reflect the onset of changes leading to icteric PBC[117]. It is clear to say that the glycosylating function of liver cells is retained at early stages of the disease.
At the late stage of the disease, hyperbilirubinemia may also develop due to erythrocytic hyperhemolysis caused by excess serum bile acids in patients with PBC. As potent detergents, bile acids (they are absent in systemic circulation in the normal state) can solubilize the cytoplasmic membranes of erythrocytes and other blood formed elements, by causing their hemolysis. This in turn leads to the development of erythrocytopenia, thrombocytopenia, hypochromic anemia, and other hematological symptoms occurring in advanced stages of the disease with the significant increase in the serum bile acid levels[118]. The degree of hyperbilirubinemia is characterized by not only the conjugated fraction of bilirubin, but also by its unconjugated fraction.
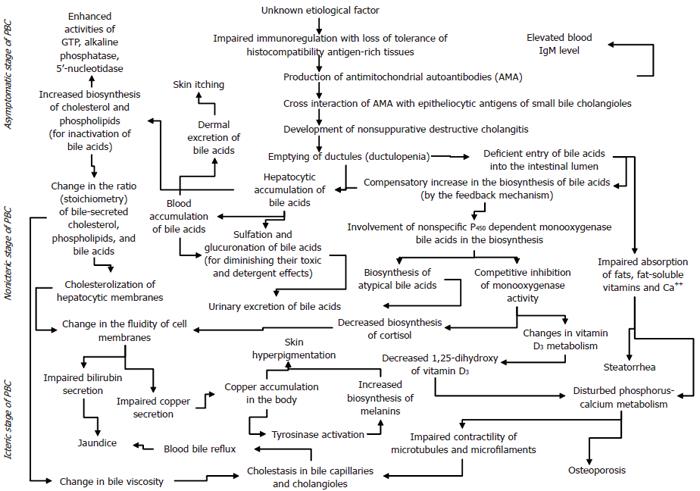
Thus, an autoimmune pathological process occurring for unknown reasons triggers the development of intrahepatic cholestasis in PBC. A gradual progression of the latter contributes to the occurrence of immunological, biochemical, morphological, and clinical signs of the disease. The mechanisms responsible for triggering and developing the major signs of PBC are given in the scheme (Figure 4).
The presented diagram is far from perfection. The diagram contains many debatable representations, but it gives an insight into the mechanism of development of major clinical signs in PBC and into their sequence and into that it is necessary to break a "closed vicious circle" in order to prevent the progression of the disease. This can be done by externally administering the missing content of bile acids into the intestinal lumen. The given scheme shows that use of ursodeoxycholic acid (UDCA) is a pathogenetically justified treatment for PBC, which promotes the stabilization of the disease process.
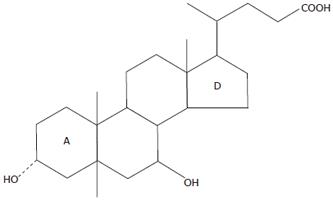
UDCA is a 7-beta-epimer of chenodeoxycholic acid (CDCA) (Figure 5). The OH group at carbonic atom 7 is at the alpha-position in CDCA, one of the primary bile acids, and that is at the beta-position in UDCA. It is precisely these structural differences that are at first glance minor, inducing essential dissimilarities of their chemical properties: UDCA is more hydrophilic and less hepatotoxic. With administration of UDCA agents, there is systemic improvement, diminished weakness, and reduced skin itch intensity. Moreover, it should be noted that some patients report greater itching in the first month of an agent’s administration and when temperature drastically drops in the transition fall-winter period. Intensified skin itching at the beginning of a drug’s administration seems to be associated with the incorporation of UDCA into enterohepatic circulation of bile acids and with their blood concentrations. However, then there is a gradual substitution of endogenous bile acids for administered UDCA and alleviation of skin itch.
The use of UDCA drugs in cholestatic liver lesions results in stabilization of clinical and biochemical signs of the disease. The stabilizing effect of UDCA in patients with PBC is associated with the delivery of missing bile acids into the small bowel (replacement therapy). The drug also inhibits the hepatocytic biosynthesis of endogenous bile acids from cholesterol. The “closed vicious circle” is discontinued. UDCA is particularly effective in early-stage[119,120] and asymptomatic PBC[121]. The early use of the drug prevents these histologic features of PBC[122]. In 30% of patients with PBC, UDCA causes full biochemical normalization, while 70% are incomplete responders[123]. In early PBC, higher dose ursodeoxycholic acid appears to be more effective than the currently recommended doses[124].
UDCA has a positive effect on the prognosis of PBC and can slow down the progression to end- stage liver disease[125]. Treatment with UDCA prevents the deposition of perisinusoidal collagen and reduces the apoptotic activity in PBC patients after 2 years of therapy[126]. Increased endothelin-2 is an early defect in PBC that is significantly reduced by UDCA treatment[127]. Budesonide combined with UDCA improves liver histology, whereas the effect of UDCA alone is mainly on laboratory values[128]. UDCA is not a relevant inducer of CYP3A enzymes in humans[129].
More significant UDCA-induced changes are observed at early stages of the disease. The use of UDCA in patients with PBC is pathogenetically substantiated. However, this treatment modality does not eliminate the cause of the disease and patients with PBC have to take UDCA agents continuously at short intervals.
There are available reports wherein the authors state that UDCA agents show a low efficacy[130,131].
Excess of endogenous blood bile acids can be eliminated by plasmapheresis, followed by a mandatory transfusion of fresh-frozen plasma of the analogous blood group and/or albumin. Plasmapheresis enhances and accelerates the positive therapeutic effect of UDCA agents.
Ion-exchange resins [cholestyramine (vasosan, quanta-lan), bilignin, questran] are used to control skin itching in patients with PBC. These drugs are not absorbed by enterocytes. By binding to bile acids in the intestinal lumen, ion-exchange resins clear them from the body by transit, by excluding from enterohepatic circulation. This causes a decrease in bile acid levels in blood and, accordingly, in the skin, which lowers the intensity of skin itching.
Ion-exchange resins may not be co-administered with UDCA agents since the latter will combine and be cleared from the body by transit, without achieving a therapeutic effect.
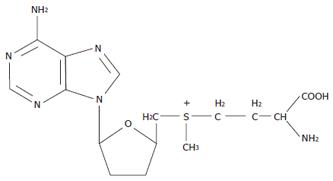
The given diagram also allows an understanding of the pathogenetic mechanisms of the efficiency of use of S-adenosyl-L-methionine in patients with PBC. S-adenosyl-L-methionine is a product of metabolism of the essential sulfur-containing aminoacid methionine (Figure 6). The latter is an officinal drug recommended for the treatment of chronic liver disease. Moreover, methionine is either built into new-synthesized proteins or metabolized to form an active form of S-adenosyl-L-methionine.
The use of S-adenosyl-L-methionine results in relieved skin itching and slightly stabilized clinical and biochemical signs of the disease. The beneficial effect of the drug on the intensity of skin itch is more pronounced when it is parenterally administered.
The mechanism of action of S-adenosyl-L-methionine in chronic cholestatic liver diseases is not completely elucidated so far. This compound is known to play an important role in the body’s cells as a source of methyl and sulfonate groups. Therefore it may be suggested that S-adenosyl-L-methionine participates in the formation of sulfated bile acids that, unlike nonsulfated ones, may be renally cleared from the body. Moreover, their content in the blood and tissues of the body may decrease, which should yield a positive therapeutic effect. S-adenosyl-L-methionine may also participate in the cellular biosynthesis of phospholipids. This may contribute to the formation of micellar-lamellar structures composed of phospholipids and bile acids. The formation of such structures enables the toxic effect of excess bile acids to be neutralized. Reasoning from these assumptions and the mechanism of development of intrahepatic cholestasis in PBC (see the scheme), the use of S-adenosyl-L-methionine in chronic liver diseases accompanied by intrahepatic cholestasis is pathogenetically justified. Its use is perhaps long and conceivably continuous, as the administration of UDCA agents.
It is advisable to co-administer UDCA and S-adenosyl-L-methionine[132]. This should lead to the potentiation of a therapeutic effect of each of the drugs individually.
Since the etiology and pathogenesis of cholestatic liver lesions remain unknown so far, their treatment is generally symptomatic and aimed at eliminating skin itching[133-137] and jaundice, diminishing steatorrhea, and simultaneously affecting osteoporosis[83,138-140]. The assumed autoimmune nature of PBC has served as the basis for using prednisolone[141] and other immunomodulators[142-144]. However, a rapid rise in bone changes, a great deal of side effects, and a low therapeutic efficacy of these agents have not permitted these drugs to be commonly used for the treatment of PBC[141].
Liver transplantation currently remains to be the most radical treatment for PBC[145,146]. However, selection of patients for surgery is a difficult task. The patient generally gives his/her consent to surgery when his/her condition causes a drastic reduction in the quality of life. Severe weakness, intolerable skin itch, and persistent severe jaundice make the patient be at home and complicate his/her relations with his/her peoples and relatives. This condition is aggravated by the occurrence of ascitis, bleedings from the varicose veins of the esophagus and stomach, or encephalopathy[147]. Liver transplantation may be expected to show the best prognosis in this condition[148,149]. As a rule, the postoperative rehabilitative period is fair[150].
After liver transplantation, there is a change in the titer of anti-mitochondrial autoantibodies: the AMA titers generally drop in the first postoperative months then tend to return to their original values[9].
Whether the disease recurs remains to be solved[151]. Histological changes typical of PBC are detectable in the needle liver biopsy specimens in 9 of 10 patients a year after surgery; of them, 4 patients are then observed to have skin itch and/or other clinical signs[152]. On the contrary, Demetris AJ et al[153] detected no histological data of a recurrence in patients with PBC after liver transplantation. Changes in the bile ducts may be induced by other causes: hepatitis C or G viruses; cytomegalovirus; a gradual rejection of the organ or problems associated with biliary anastomotic changes.
With this, 25% of patients needed liver retrans-plantation due to the development of “bile duct loss” syndrome.
In conclusion, a great body of new data on primary biliary cirrhosis has been recently accumulated. However, inadequate efficiency of its therapy has given impetus to further studies of the pathogenetic mechanisms of the disease[154] and on their basis to searches for new treatment options[155]. No specific therapy that effectively stops or reverses disease progression has been identified, thus it behooves investigators to aggressively pursue identification of the etiology of PBC[156].
Author thanks Professor TM Tzharegorodtzheva, Professor LU Ilchenko and Dr. TI Sharafanova for their collaboration in the field of PBC research.
S- Editor Liu Y L- Editor Alpini GD E- Editor Liu WF
| 1. | Loginov АС. Classification and nomenclature of chronic liver diseases. Rossiyskiy Gastroenterologicheskiy Zhurnal. 1995;2:3-8. |
| 2. | Charatcharoenwitthaya P, Lindor KD. Current concepts in the pathogenesis of primary biliary cirrhosis. Ann Hepatol. 2005;4:161-175. [PubMed] |
| 3. | Leung PS, Coppel RL, Gershwin ME. Etiology of primary biliary cirrhosis: the search for the culprit. Semin Liver Dis. 2005;25:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Sutton I, Neuberger J. Primary biliary cirrhosis: seeking the silent partner of autoimmunity. Gut. 2002;50:743-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 5. | Gershwin ME, Selmi C. Apocalypsal versus apocryphal: the role of retroviruses in primary biliary cirrhosis. Am J Gastroenterol. 2004;99:2356-2358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Mao TK, Davis PA, Odin JA, Coppel RL, Gershwin ME. Sidechain biology and the immunogenicity of PDC-E2, the major autoantigen of primary biliary cirrhosis. Hepatology. 2004;40:1241-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Inoue T, Shiraki K, Fuke H, Yamanaka Y, Miyashita K, Ito K, Suzuki M, Sugimoto K, Murata K, Nakano T. Primary biliary cirrhosis after aortoiliac reconstruction surgery using a Y-graft. World J Gastroenterol. 2005;11:6219-6220. [PubMed] |
| 8. | Amano K, Leung PS, Rieger R, Quan C, Wang X, Marik J, Suen YF, Kurth MJ, Nantz MH, Ansari AA. Chemical xenobiotics and mitochondrial autoantigens in primary biliary cirrhosis: identification of antibodies against a common environmental, cosmetic, and food additive, 2-octynoic acid. J Immunol. 2005;174:5874-5883. [PubMed] |
| 9. | Leuschner U. Primary biliary cirrhosis--presentation and diagnosis. Clin Liver Dis. 2003;7:741-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 10. | Golovnova EV, Il'chenko LIu, Tsaregorodtseva TM, Serova TI, Gudkova RV, Shepeleva SD, Tkachev VD. [Primary biliary cirrhosis: 12-year experience in observation]. Ter Arkh. 2003;75:26-30. [PubMed] |
| 11. | Prince MI, Chetwynd A, Craig WL, Metcalf JV, James OF. Asymptomatic primary biliary cirrhosis: clinical features, prognosis, and symptom progression in a large population based cohort. Gut. 2004;53:865-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 12. | Tanaka A, Miyakawa H, Luketic VA, Kaplan M, Storch WB, Gershwin ME. The diagnostic value of anti-mitochondrial antibodies, especially in primary biliary cirrhosis. Cell Mol Biol (Noisy-le-grand). 2002;48:295-299. [PubMed] |
| 13. | Sakauchi F, Mori M, Zeniya M, Toda G. Antimitochondrial antibody negative primary biliary cirrhosis in Japan: utilization of clinical data when patients applied to receive public financial aid. J Epidemiol. 2006;16:30-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Nakamura M. [The significance of anti-nuclear envelope (gp210) antibody in primary biliary cirrhosis]. Nihon Rinsho Meneki Gakkai Kaishi. 2005;28:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Zhang FK, Jia JD, Wang BE. Clinical evaluation of serum antimitochondrial antibody-negative primary biliary cirrhosis. Hepatobiliary Pancreat Dis Int. 2004;3:288-291. [PubMed] |
| 16. | Miyachi K, Shibata M, Hasegawa C, Onozuka Y, Fritzler MJ. [Case of primary biliary cirrhosis patient with anti-p97/VCP antibodies presenting a mild clinical course]. Rinsho Byori. 2005;53:19-23. [PubMed] |
| 17. | Kikuchi K, Lian ZX, Yang GX, Ansari AA, Ikehara S, Kaplan M, Miyakawa H, Coppel RL, Gershwin ME. Bacterial CpG induces hyper-IgM production in CD27(+) memory B cells in primary biliary cirrhosis. Gastroenterology. 2005;128:304-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Benson GD, Kikuchi K, Miyakawa H, Tanaka A, Watnik MR, Gershwin ME. Serial analysis of antimitochondrial antibody in patients with primary biliary cirrhosis. Clin Dev Immunol. 2004;11:129-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Mori T, Ono K, Hakozaki M, Kochi H. Epitope mapping on E1alpha subunit of pyruvate dehydrogenase complex with autoantibodies of patients with primary biliary cirrhosis. Liver Int. 2003;23:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Wenchich L, Krechler T, Horak J, Sedivá A, Bartůnková J, Hansiková H, Martásek P, Zeman J, Svestka T. [Primary biliary cirrhosis--specific anti-mitochondrial antibodies]. Vnitr Lek. 2004;50:842-845. [PubMed] |
| 21. | Mao TK, Lian ZX, Selmi C, Ichiki Y, Ashwood P, Ansari AA, Coppel RL, Shimoda S, Ishibashi H, Gershwin ME. Altered monocyte responses to defined TLR ligands in patients with primary biliary cirrhosis. Hepatology. 2005;42:802-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Amano K, Leung PS, Xu Q, Marik J, Quan C, Kurth MJ, Nantz MH, Ansari AA, Lam KS, Zeniya M. Xenobiotic-induced loss of tolerance in rabbits to the mitochondrial autoantigen of primary biliary cirrhosis is reversible. J Immunol. 2004;172:6444-6452. [PubMed] |
| 23. | Flannery GR, Burroughs AK, Butler P, Chelliah J, Hamilton-Miller J, Brumfitt W, Baum H. Antimitochondrial antibodies in primary biliary cirrhosis recognize both specific peptides and shared epitopes of the M2 family of antigens. Hepatology. 1989;10:370-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Long SA, Van de Water J, Gershwin ME. Antimitochondrial antibodies in primary biliary cirrhosis: the role of xenobiotics. Autoimmun Rev. 2002;1:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Kita H, He XS, Gershwin ME. Autoimmunity and environmental factors in the pathogenesis of primary biliary cirrhosis. Ann Med. 2004;36:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Bogdanos DP, Baum H, Grasso A, Okamoto M, Butler P, Ma Y, Rigopoulou E, Montalto P, Davies ET, Burroughs AK. Microbial mimics are major targets of crossreactivity with human pyruvate dehydrogenase in primary biliary cirrhosis. J Hepatol. 2004;40:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Bogdanos DP, Baum H, Gunsar F, Arioli D, Polymeros D, Ma Y, Burroughs AK, Vergani D. Extensive homology between the major immunodominant mitochondrial antigen in primary biliary cirrhosis and Helicobacter pylori does not lead to immunological cross-reactivity. Scand J Gastroenterol. 2004;39:981-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Selmi C, Gershwin ME. Bacteria and human autoimmunity: the case of primary biliary cirrhosis. Curr Opin Rheumatol. 2004;16:406-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Hopf U, Möller B, Stemerowicz R, Lobeck H, Rodloff A, Freudenberg M, Galanos C, Huhn D. Relation between Escherichia coli R(rough)-forms in gut, lipid A in liver, and primary biliary cirrhosis. Lancet. 1989;2:1419-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 89] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Butler P, Valle F, Hamilton-Miller JM, Brumfitt W, Baum H, Burroughs AK. M2 mitochondrial antibodies and urinary rough mutant bacteria in patients with primary biliary cirrhosis and in patients with recurrent bacteriuria. J Hepatol. 1993;17:408-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | O'Donohue J, McFarlane B, Bomford A, Yates M, Williams R. Antibodies to atypical mycobacteria in primary biliary cirrhosis. J Hepatol. 1994;21:887-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Vilagut L, Parés A, Viñas O, Vila J, Jiménez de Anta MT, Rodés J. Antibodies to mycobacterial 65-kD heat shock protein cross-react with the main mitochondrial antigens in patients with primary biliary cirrhosis. Eur J Clin Invest. 1997;27:667-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Bogdanos DP, Pares A, Baum H, Caballeria L, Rigopoulou EI, Ma Y, Burroughs AK, Rodes J, Vergani D. Disease-specific cross-reactivity between mimicking peptides of heat shock protein of Mycobacterium gordonae and dominant epitope of E2 subunit of pyruvate dehydrogenase is common in Spanish but not British patients with primary biliary cirrhosis. J Autoimmun. 2004;22:353-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Bogdanos DP, Baum H, Okamoto M, Montalto P, Sharma UC, Rigopoulou EI, Vlachogiannakos J, Ma Y, Burroughs AK, Vergani D. Primary biliary cirrhosis is characterized by IgG3 antibodies cross-reactive with the major mitochondrial autoepitope and its Lactobacillus mimic. Hepatology. 2005;42:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Selmi C, Balkwill DL, Invernizzi P, Ansari AA, Coppel RL, Podda M, Leung PS, Kenny TP, Van De Water J, Nantz MH. Patients with primary biliary cirrhosis react against a ubiquitous xenobiotic-metabolizing bacterium. Hepatology. 2003;38:1250-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 223] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 36. | Olafsson S, Gudjonsson H, Selmi C, Amano K, Invernizzi P, Podda M, Gershwin ME. Antimitochondrial antibodies and reactivity to N. aromaticivorans proteins in Icelandic patients with primary biliary cirrhosis and their relatives. Am J Gastroenterol. 2004;99:2143-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Kaplan MM. Novosphingobium aromaticivorans: a potential initiator of primary biliary cirrhosis. Am J Gastroenterol. 2004;99:2147-2149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | MacSween RNM, Anthoni PP, Schener JJ. Pathology of the liver. Edinbourgh Pub. 1979;458. |
| 39. | Loginov AS. Aruin LI Clinical morphology of the liver. Moscow: Meditsina Publishers 1985; 181. |
| 40. | Wu CT, Davis PA, Luketic VA, Gershwin ME. A review of the physiological and immunological functions of biliary epithelial cells: targets for primary biliary cirrhosis, primary sclerosing cholangitis and drug-induced ductopenias. Clin Dev Immunol. 2004;11:205-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Harada K, Ozaki S, Sudo Y, Tsuneyama K, Ohta H, Nakanuma Y. Osteopontin is involved in the formation of epithelioid granuloma and bile duct injury in primary biliary cirrhosis. Pathol Int. 2003;53:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Harada K, Isse K, Tsuneyama K, Ohta H, Nakanuma Y. Accumulating CD57 + CD3 + natural killer T cells are related to intrahepatic bile duct lesions in primary biliary cirrhosis. Liver Int. 2003;23:94-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Golovanova EV, Il'chenko LIu, Tsaregorodtseva TM, Serova TI, Gudkova RB. [Cytokines in primary biliary cirrhosis (diagnostic and prognostic value)]. Ter Arkh. 2004;76:8-11. [PubMed] |
| 44. | Harada K, Isse K, Kamihira T, Shimoda S, Nakanuma Y. Th1 cytokine-induced downregulation of PPARgamma in human biliary cells relates to cholangitis in primary biliary cirrhosis. Hepatology. 2005;41:1329-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Kita H, Nalbandian G, Keeffe EB, Coppel RL, Gershwin ME. Pathogenesis of primary biliary cirrhosis. Clin Liver Dis. 2003;7:821-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 46. | Medina J, Sanz-Cameno P, García-Buey L, Martín-Vílchez S, López-Cabrera M, Moreno-Otero R. Evidence of angiogenesis in primary biliary cirrhosis: an immunohistochemical descriptive study. J Hepatol. 2005;42:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Takii Y, Nakamura M, Ito M, Yokoyama T, Komori A, Shimizu-Yoshida Y, Nakao R, Kusumoto K, Nagaoka S, Yano K. Enhanced expression of type I interferon and toll-like receptor-3 in primary biliary cirrhosis. Lab Invest. 2005;85:908-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 48. | Hokari A, Zeniya M, Esumi H, Kawabe T, Gershwin ME, Toda G. Detection of serum nitrite and nitrate in primary biliary cirrhosis: possible role of nitric oxide in bile duct injury. J Gastroenterol Hepatol. 2002;17:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Aboutwerat A, Pemberton PW, Smith A, Burrows PC, McMahon RF, Jain SK, Warnes TW. Oxidant stress is a significant feature of primary biliary cirrhosis. Biochim Biophys Acta. 2003;1637:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 50. | Wu CT, Eiserich JP, Ansari AA, Coppel RL, Balasubramanian S, Bowlus CL, Gershwin ME, Van De Water J. Myeloperoxidase-positive inflammatory cells participate in bile duct damage in primary biliary cirrhosis through nitric oxide-mediated reactions. Hepatology. 2003;38:1018-1025. [PubMed] |
| 51. | Sasaki M, Ikeda H, Haga H, Manabe T, Nakanuma Y. Frequent cellular senescence in small bile ducts in primary biliary cirrhosis: a possible role in bile duct loss. J Pathol. 2005;205:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Saxena R, Hytiroglou P, Thung SN, Theise ND. Destruction of canals of Hering in primary biliary cirrhosis. Hum Pathol. 2002;33:983-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 53. | Yokomori H, Oda M, Yoshimura K, Nomura M, Ogi M, Wakabayashi G, Kitajima M, Ishii H. Expression of intercellular adhesion molecule-1 and lymphocyte function-associated antigen-1 protein and messenger RNA in primary biliary cirrhosis. Intern Med. 2003;42:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 54. | Yokomori H, Oda M, Ogi M, Wakabayashi G, Kawachi S, Yoshimura K, Nagai T, Kitajima M, Nomura M, Hibi T. Expression of adhesion molecules on mature cholangiocytes in canal of Hering and bile ductules in wedge biopsy samples of primary biliary cirrhosis. World J Gastroenterol. 2005;11:4382-4389. [PubMed] |
| 55. | Burt AD. Primary biliary cirrhosis and other ductopenic diseases. Clin Liver Dis. 2002;6:363-80, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Xu L, Shen Z, Guo L, Fodera B, Keogh A, Joplin R, O'Donnell B, Aitken J, Carman W, Neuberger J. Does a betaretrovirus infection trigger primary biliary cirrhosis. Proc Natl Acad Sci USA. 2003;100:8454-8459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 163] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 57. | Vierling JM. Primary biliary cirrhosis and autoimmune cholangiopathy. Clin Liver Dis. 2004;8:177-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Gentilini P, Teodori U, Gorini S, Popper H. Intrahepatic cholestasis. New-York: Raven-press Pub 1975; . |
| 59. | Lopukhin YuM, Archakov AI, Vladimirov YuA, Kogan EM. Cholesterinosis. Moscow: Meditsina Publishers 1983; . |
| 60. | Loginov AS. Intrahepatic cholestasis: a cardinal problem of modern hepatology. Collected articles “Topical aspects of gastroenterology”. М.,. 1977;3-12. |
| 61. | Zhang F, Jia J, Wang B, Qian L, Yin S, Wang Y, Cui Y, You H, Ma H, Wang H. [Clinical characteristics of primary biliary cirrhosis: a report of 45 cases]. Zhonghua Neike Zazhi. 2002;41:163-167. [PubMed] |
| 62. | Hatoff DE, Toyota N, Wong C, Miller AL, Takeya M, Miyai K. Rat liver alkaline phosphatases. Evidence hepatocyte and portal triad enzymes differ. Dig Dis Sci. 1985;30:564-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 63. | Kaplan MM, Righetti A. Induction of rat liver alkaline phosphatase: the mechanism of the serum elevation in bile duct obstruction. J Clin Invest. 1970;49:508-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 151] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 64. | Kryszewski AJ, Neale G, Whitfield JB, Moss DW. Enzyme changes in experimental biliary obstruction. Clin Chim Acta. 1973;47:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Metzler DE. Biochemistry. In three volumes. Translated from English. Moscow: Mir Publishers 1980; 119. |
| 66. | Angelico M, Alvaro D, Attili AF, and Cantafora A. Mechanisms of secretion of biliary phosphatidylcholines: the role of bile acids. Ital J Gastroenterol. 1985;17:278-281. |
| 67. | Romanenko VD. The effect of alkaline phosphatase on the biligenic function of the liver and the bile electrolyte excretion. Fiziologichniy Zhurnal. 1971;17:790-793. |
| 68. | Reshetnyak VI, Ozerova IN, Perova NV, Ilchenko LIu, Tkachev VD, Golovanova EV, Vinnickaya EV. Investigation of the lipid composition of liver cells and serum in patients with primary biliary cirrhosis (PBC). Rossiyskiy Gastroenterologicheskiy Zhurnal. 2001;2:143-144. |
| 69. | Grattagliano I, Giudetti AM, Grattagliano V, Palmieri VO, Gnoni GV, Lapadula G, Palasciano G, Vendemiale G. Structural and oxidative modifications of erythrocyte ghosts in patients with primary biliary cirrhosis: relation with the disease stage and effect of bile acid treatment. Eur J Clin Invest. 2003;33:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 70. | Yokomori H, Oda M, Wakabayashi G, Kitajima M, Yoshimura K, Nomura M, Hibi T. High expressions of caveolins on the proliferating bile ductules in primary biliary cirrhosis. World J Gastroenterol. 2005;11:3710-3713. [PubMed] |
| 71. | Jorge AD, Gutierrez LS, Jorge O, Burgos MH. Ultrastructural and cytochemical changes in the liver of primary biliary cirrhosis patients. Biocell. 2002;26:253-262. [PubMed] |
| 72. | Loginov AS, Iamskova VP, Tumanova NB, Tkachev VD, Reshetniak VI. [Study of hepatocyte adhesion in chronic diffuse diseases of the liver]. Biull Eksp Biol Med. 1989;108:160-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 73. | Sakisaka S, Kawaguchi T, Taniguchi E, Hanada S, Sasatomi K, Koga H, Harada M, Kimura R, Sata M, Sawada N. Alterations in tight junctions differ between primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology. 2001;33:1460-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Melero S, Spirlì C, Zsembery A, Medina JF, Joplin RE, Duner E, Zuin M, Neuberger JM, Prieto J, Strazzabosco M. Defective regulation of cholangiocyte Cl-/HCO3(-) and Na+/H+ exchanger activities in primary biliary cirrhosis. Hepatology. 2002;35:1513-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 75. | Nemchausky BA, Layden TJ, Boyer JL. Effects of chronic choleretic infusions of bile acids on the membrane of the bile canaliculus. A biochemical and morphologic study. Lab Invest. 1977;36:259-267. [PubMed] |
| 76. | Poupon R, Chrétien Y, Poupon RE, Ballet F, Calmus Y, Darnis F. Is ursodeoxycholic acid an effective treatment for primary biliary cirrhosis. Lancet. 1987;1:834-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 368] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 77. | Fickert P, Trauner M, Fuchsbichler A, Stumptner C, Zatloukal K, Denk H. Mallory body formation in primary biliary cirrhosis is associated with increased amounts and abnormal phosphorylation and ubiquitination of cytokeratins. J Hepatol. 2003;38:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 78. | Longo M, Crosignani A, Battezzati PM, Squarcia Giussani C, Invernizzi P, Zuin M, Podda M. Hyperlipidaemic state and cardiovascular risk in primary biliary cirrhosis. Gut. 2002;51:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 79. | Kanda T, Yokosuka O, Kojima H, Imazeki F, Nagao K, Tatsuno I, Saito Y, Saisho H. Severe hypercholesterolemia associated with primary biliary cirrhosis in a 44-year-old Japanese woman. World J Gastroenterol. 2004;10:2607-2608. [PubMed] |
| 80. | Stiehl A. Disturbances of bile acid metabolism in cholestasis. Clin Gastroenterol. 1977;6:45-67. [PubMed] |
| 81. | Markova MN. Cholestatic hperlipemia in patients with primary biliary cirrhosis. Collected Articles. "Gastroenter-ology-78". Vilnius. 1978;150-152. |
| 82. | Molostova LV. Hyperlipidemia in chronic intrahepatic cholestasis. Collected Articles. "Chronic Hepatitis ". Moscow. 1988;51-59. |
| 83. | Zollner G, Fickert P, Silbert D, Fuchsbichler A, Marschall HU, Zatloukal K, Denk H, Trauner M. Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J Hepatol. 2003;38:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 193] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 84. | Kunkel HG, Ahrens EH Jr. The relationship between serum lipids and the electrophoretic pattern, with particular reference to patients with primary biliary cirrhosis. J Clin Invest. 1949;28:1575-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 85. | Levy C, Lindor KD. Management of osteoporosis, fat-soluble vitamin deficiencies, and hyperlipidemia in primary biliary cirrhosis. Clin Liver Dis. 2003;7:901-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 86. | Zhang F, Jia J, Cui R, Wang B, Wang H. Clinical features of forty patients with primary biliary cirrhosis. Chin Med J (Engl). 2002;115:904-908. [PubMed] |
| 87. | Sherlock S, Dooley J. Diseases of the liver and biliary system. 9th ed. Oxford: Blackwell Sci Pub 1993; 44-58. |
| 88. | Bergasa NV. Pruritus and fatigue in primary biliary cirrhosis. Clin Liver Dis. 2003;7:879-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 89. | Loginov AS. Bile acid metabolism in liver disease with intrahepatic cholestasis. In: Mechanisms of resistance impairment, adaptation, and compensation. Tashkent 1976; 51-52. |
| 91. | Schoenfield LJ, Sjovall J. Bile acids on the skin of patients with pruritic hepatobiliary disease. Nature. 1967;213:93-94. [RCA] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 66] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 92. | Stiehl A. Bile salt sulphates in cholestasis. Eur J Clin Invest. 1974;4:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 107] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 93. | García-Marín JJ, González J, Esteller A. Influence of dehydrocholate on bilirubin transport into bile in the rat. Digestion. 1986;33:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 94. | Greim H, Trülzsch D, Czygan P, Rudick J, Hutterer F, Schaffner F, Popper H. Mechanism of cholestasis. 6. Bile acids in human livers with or without biliary obstruction. Gastroenterology. 1972;63:846-850. [PubMed] |
| 95. | Greim H, Trülzsch D, Roboz J, Dressler K, Czygan P, Hutterer F, Schaffner F, Popper H. Mechanism of cholestasis. 5. Bile acids in normal rat livers and in those after bile duct ligation. Gastroenterology. 1972;63:837-845. [PubMed] |
| 96. | Alme B, Bremmelgaard A, Sjovall J, Thomassen G. Complexity of the bile acid mixture in human urine. In: Advances in bile acid research. Matern S, Hackenschmidt J, Back P, Gerok W. Stuttgart, editors. New York 1974; 145-148. |
| 97. | Lino M, Binaut R, Noël LH, Patey N, Rustin P, Daniel L, Serpaggi J, Varaut A, Vanhille P, Knebelmann B. Tubulointerstitial nephritis and Fanconi syndrome in primary biliary cirrhosis. Am J Kidney Dis. 2005;46:e41-e46. [PubMed] |
| 98. | Floreani A, Paternoster D, Mega A, Farinati F, Plebani M, Baldo V, Grella P. Sex hormone profile and endometrial cancer risk in primary biliary cirrhosis: a case-control study. Eur J Obstet Gynecol Reprod Biol. 2002;103:154-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 99. | Alvaro D, Invernizzi P, Onori P, Franchitto A, De Santis A, Crosignani A, Sferra R, Ginanni-Corradini S, Mancino MG, Maggioni M. Estrogen receptors in cholangiocytes and the progression of primary biliary cirrhosis. J Hepatol. 2004;41:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 100. | Loginov AS, Reshetniak VI, Astaf'eva OV, Gavrilova AIu. [The level of steroid hormones in patients with primary biliary liver cirrhosis]. Biull Eksp Biol Med. 1990;109:237-239. [PubMed] |
| 101. | Keefee EB, Scharschmidt BF, Blankenship NM, Ockner RK. Studies of relationship among bile flow, liver plasma membrane NaK-ATPase, and membrane microviscosity in the rat. J Clin Invest. 1979;64:1590-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 138] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 102. | Moseley RH. Mechanisms of bile formation and cholestasis: clinical significance of recent experimental work. Am J Gastroenterol. 1986;81:731-735. [PubMed] |
| 103. | Phillips JR, Angulo P, Petterson T, Lindor KD. Fat-soluble vitamin levels in patients with primary biliary cirrhosis. Am J Gastroenterol. 2001;96:2745-2750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 104. | Di Bisceglie AM, Loyet M, Peters M. Long term follow up of bone mineral density in patients with primary biliary cirrhosis. Minerva Med. 2004;95:529-534. [PubMed] |
| 105. | Loginov AS, Salman MM, Sivash ES, Yarceva AM, Stavinskaya AA. Changes in the lung, gastrointestinal tract, and bones in patients with primary biliary cirrhosis (according to the data of X-ray studies). In: Topical aspects of gastroenterology. Moscow 1977; 40-44. |
| 106. | Loginov AS, Stavinskaya AA, Sivash ES. Changes in the skeletal system in primary biliary cirrhosis. Vestnik Rentgenologii I Radiologii. 1984;6:20-22. |
| 107. | Stavinskaya AA. Capacities of X-ray study in primary biliary cirrhosis. Dissertation for the academic degree of candidate of medical sciences. Moscow. 1985;. |
| 108. | Guañabens N, Parés A, Mariñoso L, Brancós MA, Piera C, Serrano S, Rivera F, Rodés J. Factors influencing the development of metabolic bone disease in primary biliary cirrhosis. Am J Gastroenterol. 1990;85:1356-1362. [PubMed] |
| 109. | Farias AQ, Gonçalves LL, Cançado EL, Seguro AC, Campos SB, Abrantes-Lemos CP, Carrilho FJ. Bone disease in primary biliary cirrhosis: lack of association with distal renal tubular acidosis. J Gastroenterol Hepatol. 2005;20:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 110. | Verma A, Maxwell JD, Ang L, Davis T, Hodges S, Northfield TC, Zaidi M, Pazianas M. Ursodeoxycholic acid enhances fractional calcium absorption in primary biliary cirrhosis. Osteoporos Int. 2002;13:677-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 111. | Lakatos PL, Bajnok E, Tornai I, Folhoffer A, Horváth A, Lakatos P, Szalay F. [Decreased bone mineral density and gene polymorphism in primary biliary cirrhosis]. Orv Hetil. 2004;145:331-336. [PubMed] |
| 112. | Pereira SP, O'Donohue J, Moniz C, Phillips MG, Abraha H, Buxton-Thomas M, Williams R. Transdermal hormone replacement therapy improves vertebral bone density in primary biliary cirrhosis: results of a 1-year controlled trial. Aliment Pharmacol Ther. 2004;19:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 113. | Szalay F, Folhoffer A, Horváth A, Csak T, Speer G, Nagy Z, Lakatos P, Horváth C, Habior A, Tornai I. Serum leptin, soluble leptin receptor, free leptin index and bone mineral density in patients with primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 2005;17:923-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 114. | García-Suárez C, Crespo J, Fernández-Gil PL, Amado JA, García-Unzueta MT, Pons Romero F. [Plasma leptin levels in patients with primary biliary cirrhosis and their relationship with degree of fibrosis]. Gastroenterol Hepatol. 2004;27:47-50. [PubMed] |
| 115. | Chavassieux P, Pastoureau P, Chapuy MC, Delmas PD, Meunier PJ. Glucocorticoid-induced inhibition of osteoblastic bone formation in ewes: a biochemical and histomorphometric study. Osteoporos Int. 1993;3:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 116. | Libanati CR, Baylink DJ. Prevention and treatment of glucocorticoid-induced osteoporosis. A pathogenetic perspective. Chest. 1992;102:1426-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 117. | Kojima H, Nies AT, König J, Hagmann W, Spring H, Uemura M, Fukui H, Keppler D. Changes in the expression and localization of hepatocellular transporters and radixin in primary biliary cirrhosis. J Hepatol. 2003;39:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 118. | Fuller SJ, Kumar P, Weltman M, Wiley JS. Autoimmune hemolysis associated with primary biliary cirrhosis responding to ursodeoxycholic acid as sole treatment. Am J Hematol. 2003;72:31-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 119. | Corpechot C, Carrat F, Bahr A, Chrétien Y, Poupon RE, Poupon R. The effect of ursodeoxycholic acid therapy on the natural course of primary biliary cirrhosis. Gastroenterology. 2005;128:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 276] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 120. | Tromm A, May B, Klein CG, Klein R, Fisseler-Eckhoff A, Griga T. Long-term response of primary biliary cirrhosis (stage I) to therapy with ursodeoxycholic acid. Hepatogastroenterology. 2005;52:753-756. [PubMed] |
| 121. | Dohmen K, Mizuta T, Nakamuta M, Shimohashi N, Ishibashi H, Yamamoto K. Fenofibrate for patients with asymptomatic primary biliary cirrhosis. World J Gastroenterol. 2004;10:894-898. [PubMed] |
| 122. | Poupon RE, Lindor KD, Parés A, Chazouillères O, Poupon R, Heathcote EJ. Combined analysis of the effect of treatment with ursodeoxycholic acid on histologic progression in primary biliary cirrhosis. J Hepatol. 2003;39:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 123. | Leuschner M, Holtmeier J, Ackermann H, Leuschner U. The influence of sulindac on patients with primary biliary cirrhosis that responds incompletely to ursodeoxycholic acid: a pilot study. Eur J Gastroenterol Hepatol. 2002;14:1369-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 124. | Roda E, Azzaroli F, Nigro G, Piazza F, Jaboli F, Ferrara F, Liva S, Giovanelli S, Miracolo A, Colecchia A. Improved liver tests and greater biliary enrichment with high dose ursodeoxycholic acid in early stage primary biliary cirrhosis. Dig Liver Dis. 2002;34:523-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 125. | Van Den Bogaert E, Francque S, Pelckmans P, Michielsen P. The use of ursodeoxycholic acid in patients with primary biliary cirrhosis: sense or nonsense. Acta Gastroenterol Belg. 2003;66:283-287. [PubMed] |
| 126. | Neuman MG, Cameron RG, Haber JA, Katz GG, Blendis LM. An electron microscopic and morphometric study of ursodeoxycholic effect in primary biliary cirrhosis. Liver. 2002;22:235-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 127. | Dimoulios P, Kolios G, Notas G, Matrella E, Xidakis C, Koulentaki M, Tsagarakis N, Kouroumalis A, Kouroumalis E. Ursodeoxycholic acid reduces increased circulating endothelin 2 in primary biliary cirrhosis. Aliment Pharmacol Ther. 2005;21:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 128. | Rautiainen H, Kärkkäinen P, Karvonen AL, Nurmi H, Pikkarainen P, Nuutinen H, Färkkilä M. Budesonide combined with UDCA to improve liver histology in primary biliary cirrhosis: a three-year randomized trial. Hepatology. 2005;41:747-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 159] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 129. | Dilger K, Denk A, Heeg MH, Beuers U. No relevant effect of ursodeoxycholic acid on cytochrome P450 3A metabolism in primary biliary cirrhosis. Hepatology. 2005;41:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 130. | Gluud C, Christensen E. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database Syst Rev. 2002;CD000551. [PubMed] |
| 131. | Ursodeoxycholic acid: a second look. Primary biliary cirrhosis: dashed hopes. Prescrire Int. 2002;11:67-69. [PubMed] |
| 132. | Avezov SA, Mansurov FKh. [Efficacy of combined administration of ursodeoxycholic acid and hepthral in the treatment of primary biliary cirrhosis]. Klin Med (Mosk). 2004;82:55-58. [PubMed] |
| 133. | Prince MI, Burt AD, Jones DE. Hepatitis and liver dysfunction with rifampicin therapy for pruritus in primary biliary cirrhosis. Gut. 2002;50:436-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 134. | Browning J, Combes B, Mayo MJ. Long-term efficacy of sertraline as a treatment for cholestatic pruritus in patients with primary biliary cirrhosis. Am J Gastroenterol. 2003;98:2736-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 135. | Talwalkar JA, Souto E, Jorgensen RA, Lindor KD. Natural history of pruritus in primary biliary cirrhosis. Clin Gastroenterol Hepatol. 2003;1:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 136. | Parés A, Cisneros L, Salmerón JM, Caballería L, Mas A, Torras A, Rodés J. Extracorporeal albumin dialysis: a procedure for prolonged relief of intractable pruritus in patients with primary biliary cirrhosis. Am J Gastroenterol. 2004;99:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 137. | ter Borg PC, van Os E, van den Broek WW, Hansen BE, van Buuren HR. Fluvoxamine for fatigue in primary biliary cirrhosis and primary sclerosing cholangitis: a randomised controlled trial [ISRCTN88246634]. BMC Gastroenterol. 2004;4:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 138. | Ormarsdóttir S, Ljunggren O, Mallmin H, Olsson R, Prytz H, Lööf L. Longitudinal bone loss in postmenopausal women with primary biliary cirrhosis and well-preserved liver function. J Intern Med. 2002;252:537-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 139. | Guañabens N, Parés A, Ros I, Alvarez L, Pons F, Caballería L, Monegal A, Martínez de Osaba MJ, Roca M, Peris P. Alendronate is more effective than etidronate for increasing bone mass in osteopenic patients with primary biliary cirrhosis. Am J Gastroenterol. 2003;98:2268-2274. [PubMed] |
| 140. | Levy C, Harnois DM, Angulo P, Jorgensen R, Lindor KD. Raloxifene improves bone mass in osteopenic women with primary biliary cirrhosis: results of a pilot study. Liver Int. 2005;25:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 141. | Prince M, Christensen E, Gluud C. Glucocorticosteroids for primary biliary cirrhosis. Cochrane Database Syst Rev. 2005;CD003778. [PubMed] |
| 142. | Lee YM, Kaplan MM. Efficacy of colchicine in patients with primary biliary cirrhosis poorly responsive to ursodiol and methotrexate. Am J Gastroenterol. 2003;98:205-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 143. | Bach N, Bodian C, Bodenheimer H, Croen E, Berk PD, Thung SN, Lindor KD, Therneau T, Schaffner F. Methotrexate therapy for primary biliary cirrhosis. Am J Gastroenterol. 2003;98:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 144. | Talwalkar JA, Angulo P, Keach JC, Petz JL, Jorgensen RA, Lindor KD. Mycophenolate mofetil for the treatment of primary biliary cirrhosis in patients with an incomplete response to ursodeoxycholic acid. J Clin Gastroenterol. 2005;39:168-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 145. | Takegoshi K, Tohyama T, Okada E. A case of advanced primary biliary cirrhosis treated with granulocyte and monocyte apheresis. Ther Apher Dial. 2003;7:468-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 146. | Cuković-Cavka S. [Primary biliary cirrhosis]. Acta Med Croatica. 2003;57:221-225. [PubMed] |
| 147. | Parés A, Rodés J. Natural history of primary biliary cirrhosis. Clin Liver Dis. 2003;7:779-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 148. | Gunson BK, Buckels JA, Elias E, McMaster P, Neuberger JM. Liver transplantation for primary biliary cirrhosis: the effect of disease stage at referral upon outcome after liver replacement. Transplant Proc. 1990;22:1503-1504. [PubMed] |
| 149. | Neuberger JM, Gunson BK, Buckels JA, Elias E, McMaster P. Referral of patients with primary biliary cirrhosis for liver transplantation. Gut. 1990;31:1069-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 150. | Sherlock S, Dooley J. Diseases of the liver and biliary system. 9th ed. Oxford: Blackwell Sci Pub 1993; 236-248. |
| 151. | Dietze O, Vogel W, Margreiter R. Primary biliary cirrhosis (PBC) after liver transplantation. Transplant Proc. 1990;22:1501-1502. [PubMed] |
| 152. | Polson RJ, Portmann B, Neuberger J, Calne RY, Williams R. Evidence for disease recurrence after liver transplantation for primary biliary cirrhosis. Clinical and histologic follow-up studies. Gastroenterology. 1989;97:715-725. [PubMed] |
| 153. | Demetris AJ, Markus BH, Esquivel C, Van Thiel DH, Saidman S, Gordon R, Makowka L, Sysyn GD, Starzl TE. Pathologic analysis of liver transplantation for primary biliary cirrhosis. Hepatology. 1988;8:939-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 154. | Mayo MJ. Primary biliary cirrhosis: the future. Clin Liver Dis. 2003;7:957-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 155. | Carithers RL Jr. Primary biliary cirrhosis: specific treatment. Clin Liver Dis. 2003;7:923-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 156. | Heathcote EJ. Primary biliary cirrhosis: historical perspective. Clin Liver Dis. 2003;7:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |