Published online Nov 7, 2006. doi: 10.3748/wjg.v12.i41.6689
Revised: August 12, 2006
Accepted: September 14, 2006
Published online: November 7, 2006
AIM: To evaluate the usefulness of denaturing high performance liquid chromatography (DHPLC) for analyzing microsatellite instability (MSI) status in stool DNA of patients with colorectal cancer.
METHODS: A total of 80 cancer tissues from patients with primary sporadic colorectal tumor (proximal cancer: 27, distal cancer: 53) and matched stool (which were employed for comparison with the tissues) were analyzed for MSI status in BAT 26. DNA samples extracted from stool were evaluated by nested polymerase chain reaction (PCR) and DHPLC for MSI analysis.
RESULTS: Six cases (7.5%) of MSI were identified in BAT 26 from 80 cancer tissues. All the stool DNA samples from patients whose cancer tissue showed MSI also displayed MSI in BAT 26.
CONCLUSION: As MSI is one of the established fecal DNA markers to screen colorectal cancer, we propose to use DHPLC for the MSI analysis in fecal DNA.
- Citation: Lim SB, Jeong SY, Kim IJ, Kim DY, Jung KH, Chang HJ, Choi HS, Sohn DK, Kang HC, Shin Y, Jang SG, Park JH, Park JG. Analysis of microsatellite instability in stool DNA of patients with colorectal cancer using denaturing high performance liquid chromatography. World J Gastroenterol 2006; 12(41): 6689-6692
- URL: https://www.wjgnet.com/1007-9327/full/v12/i41/6689.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i41.6689
Colorectal cancer, one of major causes of tumor-induced death in the Western population, becomes prevalent in Korea. The mortality rate from colorectal cancer has increased rapidly from 3.9 per 100 000 individuals in 1983 to 11.4 in 2003 in Korea[1]. Due to its orderly natural history, location within readily accessible organs, and high lifetime incidence, colorectal cancer is suitable for mass screening. It has been estimated that more than 50% of mortality due to colorectal cancer has been prevented through screening tests[2]. Fecal occult blood tests are non-invasive and useful, particularly as an adjunct to sigmoidoscopy[3]. However, the relatively high false positive rates and other problems have necessitated a search for more specific non-invasive tests. In this regard, assays for mutations in fecal DNA are particularly promising[4]. Following the initial identification of mutant K-ras in stools[5], many investigators have conducted molecular genetic analyses of stools. In view of the heterogeneity of mutations in colorectal cancer, multiple genetic targets have been screened. Dong et al[6] found that analysis of a combination of p53, BAT 26 and K-ras mutations in stools identified 71% cases of colorectal cancer. Ahlquist and colleagues[7] improved the detection sensitivity to 91% by using a panel consisting of p53, BAT 26, APC, and K-ras.
MSI is caused by a failure of the mismatch repair system to correct errors that occur during DNA replication. This phenomenon is characterized by the presence of novel alleles in DNA of cancer tissues that are absent in matched repetitive sequences in normal tissues designated ‘microsatellites’[8,9]. The MSI status has notable biological significance. For instance, colorectal cancer patients with MSI show better prognosis[9-11]. Cancer detection via microsatellite analyses has been reported for several malignancies, including renal cell carcinoma[12], uterine cervical carcinoma[13] and head and neck squamous cell carcinoma[14]. Koshiji et al[15] reported the results of MSI analysis of fecal DNA for colorectal cancer detection, while Traverso et al[16] demonstrated the practical application of the fecal BAT 26 assay with sigmoidoscopy.
In view of the importance of MSI analysis, these techniques required substantial improvement[17]. One particular concern was that the database would be limited if optimal high-throughput assays for running and interpreting microsatellite assays were not developed[18]. Thus, simple and automatic methods are required that make MSI analysis more systematic and convenient, and the many clinical approaches to cancer therapy feasible.
DHPLC has emerged as one of the most versatile technologies for the evaluation of genetic variations. The most patent advantage of this technique is the feasibility of automatic high-throughput analysis using computer-controlled systems. We have developed and reported a novel protocol for MSI analysis in cancer tissues that utilizes DHPLC [19]. In this study, we extended the protocol for MSI analysis using DHPLC to stool samples of patients with colorectal cancer.
Eighty cancer tissues, taken from 27 patients with cancer of the proximal colon (i.e., between the cecum and splenic flexure) and from 53 patients with distal cancer were analyzed. Matched stool samples from the 80 patients were also analyzed. None of the patients investigated had familial adenomatous polyposis or hereditary nonpolyposis colorectal cancer. All cancer tissues and stool were collected from the Center for Colorectal Cancer, National Cancer Center, Korea. The tumor stage was determined using the American Joint Committee on Cancer TNM system[20]. All patients gave informed consent prior to entry into the study.
Genomic DNA was extracted from surgical specimens, using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s specifications. Stool samples were obtained before commencing laxative treatment to prepare for surgery or colonoscopy. Solid stools were weighed into 10 g lots, and stored at -70°C. After defrosting, we employed a QIAamp Stool Mini Kit (QIAGEN, Hilden, Germany) for DNA preparation. DNA was extracted according to the manufacturer's instructions. Briefly, 180-220 mg of stool was prepared in a 2 mL microcentrifuge tube, and 1.6 mL Buffer ASL was added for homogenization of samples. Following centrifugation at full speed for 1 min, supernatants were transferred a new 2 mL tubes, and one InhibitEX tablet was added. The tablet was thoroughly dissolved in the stool solution, followed by a second round of centrifugation for 3 min. The supernatant was transferred to a new 1.5 mL tube with 25 μL of proteinase K. AL buffer (600 μL) was added to the prepared solution, and incubated at 70°C for 10 min. After the addition of 600 μL of ethanol, solutions (600 μL) were applied to the QIAamp spin column, and centrifuged three times. The column was washed with 500 μL of AW1 and AW2 and stool DNA samples eluted using 200 μL of AE buffer.
Primer sequences for amplifying microsatellite marker, BAT 26 were obtained from the GDB Human Genome Database (http://www.gdb.org). Stool DNA was initially amplified with the outer primers, forward, 5’-TTTAGGTTGCAGTTTCATCA-3’, and reverse, 5’-ACCATTCAACATTTTTAACCC-3’. The outer PCR product (1 μL) was employed for a second inner round of amplification. Inner PCR primers for BAT 26 were follows as: forward primer, 5’-ACTGTCTGCGGTAATCAAGT-3’; and reverse primer, 5’-CCATTCAACATTTTTAACCC-3’. Amplification for DHPLC analysis was performed with 1 μL of the first PCR product, 1X PCR buffer supplemented with 1.5 m mol/L MgCl2, 10 pmol/μL of primers, 50 μmol/L dNTPs (each), and 0.25 U of Taq polymerase (QIAGEN, Hilden, Germany) in a total volume of 25 μL. For heteroduplex formation, crude PCR products were denatured at 95°C for 5 min, followed by gradual cooling from 95°C to 25°C over a period of 1 h.
DHPLC was performed with a fully automated system (WAVE, Transgenomic Inc., Omaha, NE, USA) as previously reported[19]. Amplified products were automatically injected into a DNA Seq® cartridge (Transgenomic Inc., Omaha, NE, USA), and eluted at a flow rate of 0.9 mL/min through a linear gradient of acetonitrile with 0.1 mol/L triethylammonium acetate (TEAA). Buffers A (0.1 mol/L triethylammonium acetate (TEAA) solution) and B (0.1 mol/L TEAA containing 25% acetonitrile) were automatically adjusted to obtain the best running conditions. The portion of Buffer A was 53% and Buffer B was 47%. The sample injection volume per analysis was 5 μL of PCR product. Regardless of the markers and samples, the running temperature was set to 50°C for double-stranded DNA analysis in WAVEMAKERTM software (Transgenomic Inc., Omaha, NE, USA). The analysis of individual samples took an average of 9 min from sample injection to the finished result. UV detection was performed at 260 nm. DHPLC buffer conditions for the marker were automatically calculated. MSI analysis by DHPLC took 9 min per amplified DNA sample. Since no matched normal tissue DNA was required for quasimonomorphic markers of BAT 26, the MSI status was determined within 9 min. Results were analyzed using WAVEMAKER software (Transgenomic, Omaha, NE, USA.) in real time. No additional adducts (i.e., radioisotope or fluorescent-labeled primers) were employed other than the conventional PCR reaction, and no extra software was required.
Statistical analysis was performed using the Pearson’s Chi-square test, Fisher’s exact test, or Student’s t test, depending on the nature of the data. Two-tailed P < 0.05 was considered statistically significant.
We evaluated the status of the microsatellite from surgical specimens of all 80 cancers by DHPLC. DNA of adequate quality was recovered from all the lesions. We identified six cases (7.5%) of MSI in BAT 26 from 80 cancer tissues, 4 of 27 proximal colon cancers and 2 of 53 distal colorectal cancers.
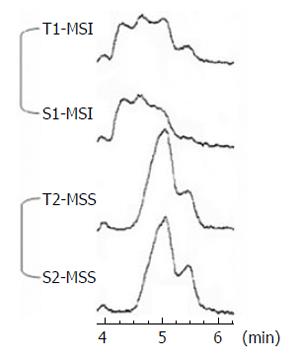
To determine the degree of concordance between BAT 26 alterations in cancer tissue and those in matched fecal DNA, we evaluated the status of the microsatellite from matched stool DNA samples of all 80 patients by nested PCR and DHPLC analysis. Seven cases of MSI in BAT 26 were identified from 80 stool DNA samples. Among 7 stool DNA samples with MSI, 6 stool samples corresponded to tissue MSI and the size of the BAT 26 alteration in the tumor and fecal DNA was identical in each patient (Figure 1). Cancer tissue from one proximal colon cancer did not display MSI, but displayed MSI repeatedly in the matched stool DNA.
MSI induced as a result of failure of the mismatch repair system has significant clinical importance in the diagnosis and prognosis of colorectal cancer. Radioactive gel-based electrophoresis and fluorescent-labeled sequencing analysis have been used for MSI analysis. However, these methods have critical limitations for use in high-throughput analysis in a clinical setting. Thus, there is a need for improved MSI analysis techniques. We have developed a novel protocol for MSI analysis in cancer tissues that utilizes DHPLC[19]. In the proposed method, standardized experimental conditions facilitate robust, high-throughput MSI analysis, and consistent results are obtained with high specificity, regardless of sample type or individual researchers’ skills. DHPLC can separate PCR products that differ by as little as a few base pairs[21]. This allows the size-dependent separation of double-stranded DNA fragments. In cases where there is a deletion in a microsatellite leading to a shorter allele, the resulting chromatogram is shifted to the left compared with that of the original allele. All the MSI samples in this investigation displayed left-shifted bands, indicating deletion alterations. The main advantage of DHPLC is that automatic high-throughput analysis is feasible using its computer-controlled systems[21].
The BAT 26 marker is employed as an indicator of microsatellite instability, since its mononucleotide tract is altered in nearly all mismatch-deficient tumors[22]. We initially attempted to amplify stool DNA using original BAT 26 markers, but failed to detect mutated DNA in DHPLC. Consequently, we devised a nested PCR analysis method by adding an outer amplification step. A comparison of the outer and inner PCR experiments disclosed that outer PCR followed by BAT 26 amplification led to the best results. Regarding the correlation between MSI status of stools and clinical data, MSI (+) status has been associated with proximal location[23]. Consistent with this, MSI (+) stools were significantly associated with proximal location in our data (P = 0.040).
Our data confirm the reliability of stool DNA for MSI analysis of colorectal cancers. Significantly, all 6 cases with BAT 26 mutations in their tissues displayed a positive stool DNA test. In one case with only MSI in stool DNA, it was possible that tumor heterogeneity influenced the result of tissue MSI. We used a part of tissue instead of whole tumor. Baisse et al[24] suggested that a molecular heterogeneity in tumors could modify MSI detection.
Main limitation of stool MSI was a low sensitivity. Colorectal cancers with MSI are less frequent even in the proximal colon compared with those without MSI. Using only MSI analysis is not suitable as a screening method. An analysis of a combination of several DNA alterations including MSI status using BAT 26 in stools improved the sensitivity over 90%[7].
In this work, we have extended the DHPLC method for MSI analysis to stool DNA and verified the reliability of stool DNA in the MSI analysis for one of the multiple markers of the genetic screening of colorectal cancers. As MSI is one of the established fecal DNA markers to screen colorectal cancer, we propose to use DHPLC for the MSI analysis in stool DNA.
S- Editor Liu Y L- Editor Ma JY E- Editor Ma WH
| 1. | Available from: http: //kosis.nso.go.kr. |
| 2. | Walsh JM, Terdiman JP. Colorectal cancer screening: clinical applications. JAMA. 2003;289:1297-1302. [RCA] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000;284:1954-1961. [RCA] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 420] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 4. | Ahlquist DA, Shuber AP. Stool screening for colorectal cancer: evolution from occult blood to molecular markers. Clin Chim Acta. 2002;315:157-168. [RCA] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Sidransky D, Tokino T, Hamilton SR, Kinzler KW, Levin B, Frost P, Vogelstein B. Identification of ras oncogene mutations in the stool of patients with curable colorectal tumors. Science. 1992;256:102-105. [RCA] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 475] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 6. | Dong SM, Traverso G, Johnson C, Geng L, Favis R, Boynton K, Hibi K, Goodman SN, D'Allessio M, Paty P. Detecting colorectal cancer in stool with the use of multiple genetic targets. J Natl Cancer Inst. 2001;93:858-865. [RCA] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 224] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Ahlquist DA, Skoletsky JE, Boynton KA, Harrington JJ, Mahoney DW, Pierceall WE, Thibodeau SN, Shuber AP. Colorectal cancer screening by detection of altered human DNA in stool: feasibility of a multitarget assay panel. Gastroenterology. 2000;119:1219-1227. [RCA] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 352] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 8. | Aaltonen LA, Peltomaki P, Leach FS, Sistonen P, Pylkkanen L, Mecklin JP, Jarvinen H, Powell SM, Jen J, Hamilton SR. Clues to the pathogenesis of familial colorectal cancer. Science. 1993;260:812-816. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1810] [Cited by in RCA: 1814] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 9. | Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816-819. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2062] [Cited by in RCA: 2059] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 10. | Samowitz WS, Curtin K, Ma KN, Schaffer D, Coleman LW, Leppert M, Slattery ML. Microsatellite instability in sporadic colon cancer is associated with an improved prognosis at the population level. Cancer Epidemiol Biomarkers Prev. 2001;10:917-923. [PubMed] |
| 11. | Lim SB, Jeong SY, Lee MR, Ku JL, Shin YK, Kim WH, Park JG. Prognostic significance of microsatellite instability in sporadic colorectal cancer. Int J Colorectal Dis. 2004;19:533-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Eisenberger CF, Schoenberg M, Enger C, Hortopan S, Shah S, Chow NH, Marshall FF, Sidransky D. Diagnosis of renal cancer by molecular urinalysis. J Natl Cancer Inst. 1999;91:2028-2032. [RCA] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Rha SH, Dong SM, Jen J, Nicol T, Sidransky D. Molecular detection of cervical intraepithelial neoplasia and cervical carcinoma by microsatellite analysis of Papanicolaou smears. Int J Cancer. 2001;93:424-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Spafford MF, Koch WM, Reed AL, Califano JA, Xu LH, Eisenberger CF, Yip L, Leong PL, Wu L, Liu SX. Detection of head and neck squamous cell carcinoma among exfoliated oral mucosal cells by microsatellite analysis. Clin Cancer Res. 2001;7:607-612. [PubMed] |
| 15. | Koshiji M, Yonekura Y, Saito T, Yoshioka K. Microsatellite analysis of fecal DNA for colorectal cancer detection. J Surg Oncol. 2002;80:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Traverso G, Shuber A, Olsson L, Levin B, Johnson C, Hamilton SR, Boynton K, Kinzler KW, Vogelstein B. Detection of proximal colorectal cancers through analysis of faecal DNA. Lancet. 2002;359:403-404. [RCA] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Sood AK, Holmes R, Hendrix MJ, Buller RE. Application of the National Cancer Institute international criteria for determination of microsatellite instability in ovarian cancer. Cancer Res. 2001;61:4371-4374. [PubMed] |
| 18. | Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248-5257. [PubMed] |
| 19. | Kim IJ, Shin Y, Kang HC, Park JH, Ku JL, Park HW, Park HR, Lim SB, Jeong SY, Kim WH. Robust microsatellite instability (MSI) analysis by denaturing high-performance liquid chromatography (DHPLC). J Hum Genet. 2003;48:525-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M. AJCC Cancer Staging Manual. 6th ed. New York: Springer-Verlag 2002; . [DOI] [Full Text] |
| 21. | Kleymenova E, Walker CL. Determination of loss of heterozygosity in frozen and paraffin embedded tumors by denaturating high-performance liquid chromatography (DHPLC). J Biochem Biophys Methods. 2001;47:83-90. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Loukola A, Eklin K, Laiho P, Salovaara R, Kristo P, Järvinen H, Mecklin JP, Launonen V, Aaltonen LA. Microsatellite marker analysis in screening for hereditary nonpolyposis colorectal cancer (HNPCC). Cancer Res. 2001;61:4545-4549. [PubMed] |
| 23. | Kazama Y, Watanabe T, Kanazawa T, Tada T, Tanaka J, Nagawa H. Mucinous carcinomas of the colon and rectum show higher rates of microsatellite instability and lower rates of chromosomal instability: a study matched for T classification and tumor location. Cancer. 2005;103:2023-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Baisse B, Bouzourene H, Saraga EP, Bosman FT, Benhattar J. Intratumor genetic heterogeneity in advanced human colorectal adenocarcinoma. Int J Cancer. 2001;93:346-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |