Published online Jan 28, 2006. doi: 10.3748/wjg.v12.i4.588
Revised: June 28, 2005
Accepted: August 3, 2005
Published online: January 28, 2006
AIM: Portal hypertension is a common complication of liver cirrhosis. Intrahepatic pressure can be elevated in several ways. Abnormal architecture affecting the vasculature, an increase in vasoconstrictors and increased circulation from the splanchnic viscera into the portal system may all contribute. It follows that endogenous vasodilators may be able to alleviate the hypertension. We therefore aimed to investigate the levels of endogenous vasodilators, nitric oxide (NO) and carbon monoxide (CO) through the expression of nitric oxide synthase (NOS) and heme oxygenase (HO).
METHOD: Cirrhotic (n = 20) and non-cirrhotic (n = 20) livers were obtained from patients who had undergone surgery. The mRNA and protein expressions of the various isoforms of NOS and HO were examined using competitive PCR, Western Blot and immunohistochemistry.
RESULTS: There was no significant change in either inducible NOS (iNOS) or neuronal NOS (nNOS) expressions while endothelial NOS (eNOS) was up-regulated in cirrhotic livers. Concomitantly, caveolin-1, an established down-regulator of eNOS, was up-regulated. Inducible HO-1 and constitutive HO-2 were found to show increased expression in cirrhotic livers albeit in different localizations.
CONCLUSION: The differences of NOS expression might be due to their differing roles in maintaining liver homeostasis and/or involvement in the pathology of cirrhosis. Sheer stress within the hypertensive liver may induce increased expression of eNOS. In turn, caveolin-1 is also increased. Whether this serves as a defense mechanism against further cirrhosis or is a consequence of cirrhosis, is yet unknown. The elevated expression of HO-1 and HO-2 suggest that CO may compensate in its role as a vasodilator albeit weakly. It is possible that CO and NO have parallel or coordinated functions within the liver and may work antagonistically in the pathophysiology of portal hypertension.
- Citation: Goh BJ, Tan BT, Hon WM, Lee KH, Khoo HE. Nitric oxide synthase and heme oxygenase expressions in human liver cirrhosis. World J Gastroenterol 2006; 12(4): 588-594
- URL: https://www.wjgnet.com/1007-9327/full/v12/i4/588.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i4.588
Liver cirrhosis is invariably associated with the incidence of hemodynamic disturbances manifested as portal hypertension and concomitant splanchnic vasodilation. Portal hypertension can be seen as a function of Ohm’s law[1]: ΔPa = Q x R, where ΔPa is the intrahepatic pressure, Q is the blood flow from systemic circulation and R is the intrahepatic vascular resistance. Increasing either or both Q and R results in an elevation of portal pressure.
Increased hepatic vascular resistance is thought to be a consequence of architectural abnormalities comprising of vascularized connective tissue which interrupt the normal organization of liver parenchyma. This establishes vascular shunts between afferent (portal and hepatic artery) and efferent (hepatic vein) vessels[2] leading to shunting. This condition is aggravated by the accommodation of the portal system to whatever is provided by the vasodilated splanchnic viscera.
Endogenous vasoconstrictors, such as endothelin and angiotensin II, mobilized by the onset or progression of cirrhosis may contribute to the increased hepatic vascular tone by the activation of hepatic stellate cells to a more contractile state[1]. It follows that mitigation of the vascular dysfunction may be promoted by endogenous vasodilators, including nitric oxide (NO) and carbon monoxide (CO).
NO has an amazing repertoire of physiological functions for a molecule with a half-life <5 s, including homeostasis and immunological defense mechanisms, within the liver. NO is synthesized from L-arginine by three known isoforms of nitric oxide synthase (NOS), namely neuronal NOS (nNOS), endothelial NOS (eNOS) and inducible NOS (iNOS)[1-4]. Under normal physiological conditions, only constitutively expressed eNOS is believed to be active in the liver and its low level of NO production suffices for regulating hepatic perfusion, preventing platelet adhesion, thrombosis and polymononuclear cell accumulation. In pathological conditions, such as sepsis, hepatitis and endotoxaemia, iNOS is activated and induced to produce sustained amounts of NO[3].
Zakhary et al[5] has suggested that CO and NO share contiguous, if not overlapping, areas of influence on vascular tone. Analogous to NOS, haem oxygenase (HO) exists in 3 isoforms: inducible HO (HO-1) and the 2 constitutively expressed HO (HO-2 and HO-3). CO, arising from the metabolism of haem by HO, has been found to attenuate sinusoidal tone and is responsible for the perfusion maintenance in normal liver[5].
Current understanding of the contributions to the onset and/or progression of cirrhosis by NOS is not clear. Accordingly, this study aims to investigate the levels of NO and CO indirectly through the expression and distribution of NOS and HO in cirrhotic and non-cirrhotic human livers.
Liver specimens were collected from 20 patients, including 10 males and 10 females (mean age 32.5 ± 25.5 years), who received orthotopic liver transplantation for end-stage liver diseases. Postoperative pathology reports confirmed the presence of cirrhosis. Samples with cirrhosis due to biliary obstruction or hepatic venous obstruction and not viral or alcoholic origin were studied. Twenty control liver tissues from 14 male and 6 female patients (mean age 57.2 ± 11.9 years) were obtained from partial hepatectomy surgery for malignant or other liver diseases (hepatocellular carcinoma, adenocarcinoma, leiomyosarcoma, gall bladder cancer infiltrating the liver, colonic carcinoma, Klaskin tumor). These controls were obtained from the outermost region of the tumor-free periphery from the excised lobe. The samples in our control group were ascertained to be cirrhosis-free.
Each patient provided a written informed consent before recruitment into the study. The protocol conformed to the ethical guidelines of the 1987 Declaration of Helsinki and those of the NMEC (National Medical Ethics Committee) - MOH (Ministry of Health) Ethical Guidelines on Research involving Human Subjects (1997).
All liver tissue samples were obtained intra-operatively and were snap frozen in liquid nitrogen not more than 20 min after excision and stored at -80 °C until processed. Venous blood of patients was collected in EDTA-tubes and centrifuged at 3 000 g for 10 min at 4 °C. Plasma samples were aliquoted and stored at -20 °C until use.
Plasma was analyzed for total NO metabolites using Total NO kit (R & D Systems, Germany). Briefly, plasma was deproteinized by ultra-filtration. Enzymatic conversion of nitrate to nitrite by nitrate reductase preceded a colorimetric detection of nitrite as an azo dye product of the Griess reaction. Absorbance was read at 570 nm.
Total RNA was extracted using TriZol Reagent (Invitrogen, Grand Island NY.), according to the manufacturer’s protocol. Reverse transcription (RT) was performed with oligo(dT) using Superscript II RNase H-reverse transcriptase (Invitrogen, Maryland). First strand cDNA synthesis was performed at 42 °C for 1 h. RNA-cDNA hybrids were denatured at 72°C for 15 min. RT-products were stored at -20 °C until use. PCR was performed using a DNA amplification kit (Qiagen, Germany) with Hybaid PCR express thermal cycler (UK).
Primer sequences were as follows: β-actin, 5’-GTGGGGCGCCCCAGGCACCA-3’ (sense) and 5’-CTCCTTAATGTCACGCACGATTC-3’ (antisense); iNOS, 5’-GTGGTCCAACCTGCAGGTCT-3’ (sense) and 5’-CATAGCGGATGAGCTGAGCATT-3’ (antisense); eNOS, 5’-GACATTGAGAGCAAAGGGCTGC-3’ (sense) and 5’-CGGCTTGTCACCTCCTGG-3’ (antisense); nNOS, 5’-GCAACAGCGGCAATTTG-3’ (sense) and 5’-GGTTGTCATCCCTCATCCGGCTG-3’ (antisense); HO-1, 5’-CAGGCAGAGAATGCTGAGTTC-3’ (sense) and 5’-GCTTCACATAGCGCTGCA-3’ (antisense); HO-2, 5’-GCAATGTCAGCGGAAGTGGAA-3’ (sense) and 5’-AAGTCACCTGAGGTGGTAGTT-3’ (antisense); caveolin-1, 5’-CATCCCGATGGCACTCATCTG-3’ (sense) and 5’-CACGGCTGATGCACTGAATCT-3’ (antisense).
Predicted sizes for PCR products were 560 bp for β-actin, 187 bp for iNOS, 510 bp for eNOS, 387 bp for nNOS, 270 bp for HO-1, 1135 bp for HO-2 and 116 bp for caveolin-1. Each 25 μL of PCR reaction contained 0.4 μmol/L of sense- and antisense primers, 2.5 μmol/L of each dNTP, 0.5 units of Taq polymerase and 2.5 μL of cDNA, except for iNOS in which 5 μL was used. Annealing temperatures for β-actin, iNOS, eNOS, nNOS, HO-1, HO-1 and caveolin-1 were 55 °C (25 cycles), 62 °C (40 cycles), 60 °C (30 cycles), 60 °C (40 cycles), 62 °C (30 cycles), 65 °C (25 cycles) and 62 °C (40 cycles), respectively. β-actin was used as a housekeeping gene.
Relative levels of mRNA expression can be semi-quantified by co-amplifying the cDNA with a known concentration of an internal competitor DNA. The competitor DNA, Mimic, is a non-homologous DNA fragment derived from the v-erb b gene to which a pair of gene-specific primers template had been added. The Mimic was constructed according to manufacturer’s protocol (Clontech Laboratories, USA). A constant amount of cDNA was first amplified with a series of 10-fold dilution of its Mimic. This titration provided a fine-tuned 2-fold dilution series of Mimic. The amount of Mimic that obtained an optical density of 1:1 was then chosen to co-amplify with all the samples. After PCR, the products were resolved on a 18 g/L agarose gel and visualized by ethidium bromide. The ratio of optical density of target against Mimic was then determined via the KODAK 1D Image Analysis Software (United States).
The expressions of NOS and HO isoforms in liver samples were measured by densitometry quantitation of immunoblots. Liver tissues were homogenized in a lysis buffer (0.5 g/L SDS in 0.1 mol/L Tris-HCl, pH 7.4) containing Complete Mini-tab EDTA proteinase inhibitor (Roche Biochemicals) and centrifuged at 13 000 g for 15 min at 4 °C. Then 100 μg of total protein was resolved on 80 g/L SDS-PAGE and transferred onto a PVDF-membrane using Mini Trans-Blot cell (BioRad Laboratories, California). Membranes were then blocked in 5% skim milk in phosphate buffered saline for 1 h at room temperature. After an overnight incubation at 4 °C using diluted monoclonal antibodies from Transduction Lab, (200x anti-iNOS, 1000x anti-eNOS, 2000x anti-nNOS, 1000x anti-HO-1 and anti-HO-2 and 500x anti-caveolin-1), the membranes were incubated with 1 000x dilution of horseradish peroxidase-conjugated secondary antibody (Amersham Pharmacia Biotech). The blots were then visualized using ECL Plus Kit (Amersham) according the manufacturer’s instructions. Relative densities of the bands were analyzed using Kodak 1D Imaging System.
Formalin-fixed and paraffin-embedded 4-μm thick sections of liver specimens were de-paraffinized in xylene (Merck, Germany) and rehydrated in decreasing concentration of ethanol. Antigen retrieval was carried out by heating the sections in 0.01 mol/L sodium citrate (pH 6.0). The sections were allowed to cool to room temperature before proceeding. Peroxidase Block (Amersham Pharmacia Biotech) was applied to inhibit endogenous peroxidase activity. Primary antibodies (same as those used in Western Immunoblot) were diluted (iNOS, eNOS, nNOS: 600x; HO-1, HO-2: 200x; caveolin-1: 400x) and incubated with the samples for 45 min at room temperature, followed by incubation with secondary antibody for 1 h at room temperature. Sections were then visualized with 3,3’-diaminobenzidine (DAB) and counter-stained with Harris’ hematoxylin. The slides were dehydrated, cleared and mounted for viewing.
Graphpad Prism 4.0 was used in all statistical analysis. Student’s t test for unpaired samples was used to compare data between cirrhotic and non-cirrhotic groups. Differences were considered statistically significant at values of P < 0.05.
Histopathology revealed micro- or macro-nodular cirrhotic morphology. Biliary cirrhosis secondary to biliary atresia or extrahepatic biliary obstruction resulting in cholestasis were the main causes of cirrhosis in our study group. The control group did not present cirrhotic morphology. All samples studied were found to be negative for hepatitis B antigen.
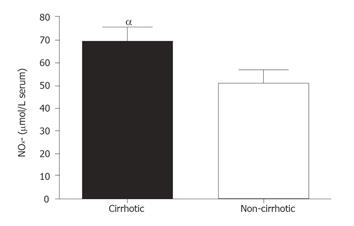
Comparison of total NOx- in systemic plasma is shown in Figure 1. The data showed that cirrhotic patients had significantly higher NOx- levels (69.72 ± 6.31 µmol/L) as compared to the controls (50.83 ± 5.67 µmol/L) (P < 0.05).
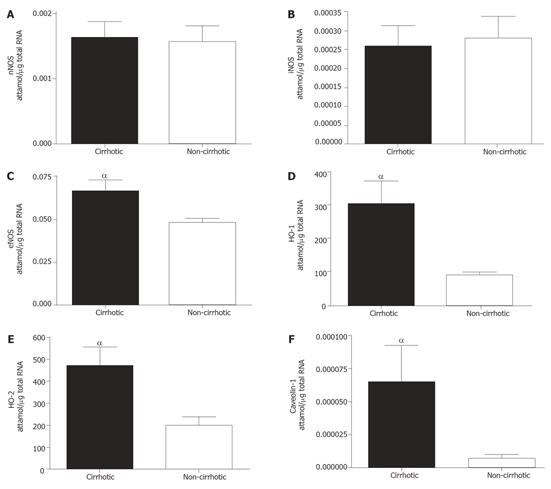
The mRNA levels of the various isoforms of NOS and HO were differentially expressed in cirrhotic and control groups (Figure 2). mRNA levels of nNOS and iNOS were not significantly different between the study and control groups (Figures 2A and 2B). Conversely, eNOS (Figure 2, P < 0.05) and both isoforms of HO (Figure 2D, HO-1: P < 0.01; Figure 2E, HO-2: P < 0.01) were significantly elevated in the cirrhotic group. Caveolin-1 mRNA expression was also significantly elevated (P < 0.05) in cirrhotic tissue (Figure 2F).
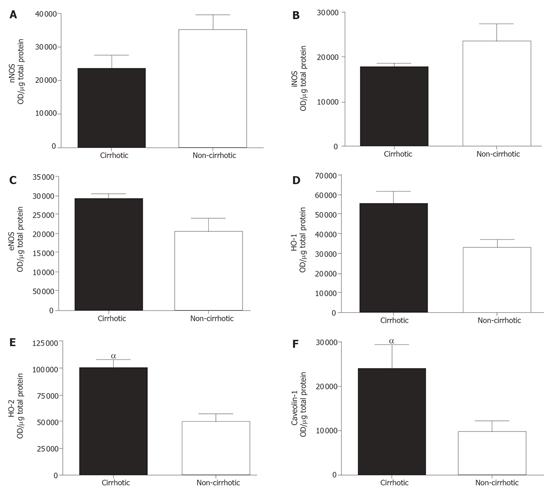
Protein levels, as semi-quantitated via densitometry of Western blot, agreed with our mRNA expression data. As shown in Figure 3A, nNOS was similar for both. Although iNOS seemed down-regulated in cirrhotic livers, it was not statistically significant (Figure 3B). We found significantly increased levels of eNOS (Figure 3C, P < 0.05), HO-1 (Figure 3D, P < 0.05) and HO-2 (Figure 3E, P < 0.001) in cirrhotic groups as compared to the control group. Likewise, caveolin-1 protein level was markedly increased (P < 0.05) in cirrhotic livers (Figure 3F).
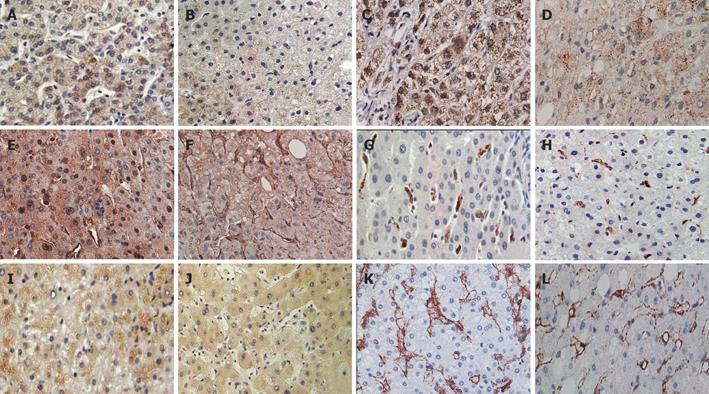
Figure 4 shows liver sections of the cirrhotic study group (left panel) and the control group (right panel). nNOS (Figures 4A and 4B) was seen to have little or no variance in intensity and localization as compared to liver sections of the cirrhotic (Figure 4A) and non-cirrhotic (Figure 4B) groups. Generally, a uniform cytoplasmic stain was found in hepatocytes, while the Kupffer and sinusoidal cells remained unstained.
Similarly, iNOS (Figures 4C and 4D) was found in cytoplasmic regions of the hepatocytes. Kupffer cells, resident macrophages of the liver, were surprisingly not stained. Overall, the cirrhotic group (Figure 4C) was ascertained to be comparable to the non-cirrhotic (Figure 4D) samples.
Conversely, eNOS (Figures 4E and 4fF) displayed a distinct difference in localization in cirrhotic tissues. In the non-cirrhotic group (Figure 4F), eNOS was localized typically in endothelial cells as well as the cytoplasm of hepatocytes. Interestingly, translocation of eNOS from the cytoplasm to nuclei was evident in cirrhotic liver sections (Figure 4E). Also, noteworthy was the apparent attenuation of eNOS expression in endothelial cells, and at the same time enhanced staining was noted in hepatocytes in cirrhotic sections. Kupffer cells exhibited strong immunoreactivity to HO-1 in cirrhotic liver sections (Figure 4G), while control groups (Figure 4H) showed less intense immunoreactivity. HO-1 staining in Kupffer cells was noted to have a higher intensity in cirrhotic liver specimens compared to non-cirrhotic specimens. In contrast, HO-2 (Figures 4I and 4J) was found in the cytoplasm of hepatocytes. Akin to HO-1, HO-2 was expressed at a higher level in cirrhotic liver as compared to the controls.
Moreover, we observed that caveolin-1 was more vividly stained in the study group (Figure 4K). They shared the same localization as that in the non-cirrhotic group (Figure 4L), namely the endothelial cells.
Several studies on diverse cirrhotic animal models have not reached agreement as to which isoform of NOS is the progressive/causative agent in portal hypertension[6-8]. Studies on rat models have found a marked increase in iNOS mRNA and protein expression[6-8], but upon treatment with specific eNOS inhibitors, injury exacerbation can be observed in spite of increased levels of iNOS[9]. Ineffective selective inhibition of iNOS in ameliorating portal pressure underlined the importance of eNOS in intrahepatic haemodynamics[6-9].
Similar to our results, McNaughton et al[10] found no change in iNOS expression. Mohammed et al[11] found that both eNOS and iNOS levels were elevated in cirrhosis, and systemic plasma NOx- increase in cirrhosis was mainly attributed to iNOS induction. Our data showed a similar increase in venous plasma NOx-. We hesitate to attribute this to increased NOS in the liver, although it remains a plausible source of NO.
In our patient group, eNOS and caveolin-1 were found to have greater expression, while no substantial changes in iNOS or nNOS were found. This induction of eNOS can be attributed to the shear stresses resulting from hypertension[1,3]. Previously, we demonstrated that eNOS mRNA and protein levels in bile duct-ligated (BDL) rats were increased, yet activity declined by more than half[12]. In support of this, Sarela et al[13] showed that constitutive NOS activity was substantially lower in cirrhotic liver. In addition to the Ca2+/calmodulin-dependent regulation, caveolin-1 has been found to bind and inactivate NO production by eNOS[14,15]. Caveolin-1 is known to be stimulated by cholesterol receptors[14,15]. It is possible that the abnormal structure of the cirrhotic liver causes an induction of caveolin-1 or that the increasing fatty depositions common in cirrhosis contributes to this.
At rest, eNOS is bound to caveolin-1 and inactive. Upon stimulation, for example by shear stress, eNOS uncouples from caveolin-1 and translocates to the plasma membrane where it releases NO[14]. Shear stress within the sinusoids of the cirrhotic liver presents unremitting stimuli for eNOS activation; which in turn is inhibited by increased caveolin-1 expression, reducing NO production intrahepatically. Our findings corroborate those of Yokomori et al[15] that eNOS and caveolin-1 expressions increase in cirrhotic patients.
Not exclusive to endothelial cells, eNOS has been found in a variety of cell types, including mesangial cells, neurons[8] and hepatocytes[10]. We obviously showed that heptocellular cytoplasmic localization of eNOS was not unique to cirrhotic livers, albeit the endothelial cells stained more intensely in non-cirrhotic livers (Figures 4E and 4F). This was also pointed out by McNaughton et al[10] where a similar translocation of eNOS to the nuclear periphery in cirrhotic sections was highlighted. The apparent attenuation of eNOS in cirrhotic endothelial cells may be due to the increased caveolin-1 expression in the same site.
In normal liver, HO is accountable for the perfusion maintenance and sinusoidal tone[5]. Our study showed that HO-1 and HO-2 had increased expression in cirrhotic livers. HO-1 seems to be exclusively expressed in Kupffer cells while HO-2 can be visualized in the hepatocyte cytoplasm. This was also evident in BDL rat, where HO-1 showed increased mRNA and protein levels[16]. This corroborates results from Makino et al[17] as they found that HO-1 activity was markedly elevated in cirrhotic livers and inferred that the cirrhotic liver up-regulated HO activity mainly through the inducible isoform, HO-1. Induction of HO has also been attributed to shear stress[17] and in the context of portal hypertension, up-regulation of HO-1 in Kupffer cells may serve as a local sensor of hemodynamics. In a study conducted by Sacerdoti et al[18] using CCl4-induced cirrhosis in rats, HO-2 but not HO-1 was responsible for the splanchnic vascular hyporeactivity to vasoconstrictors. In our study, HO-2 was moderately increased in cirrhotic livers. However, the role of HO-2 in the pathophysiology of cirrhosis has not been well understood as yet. Our data suggest that it may have a complementary function to that of HO-1 within the cirrhotic liver. Yet its obviously different locale from HO-1 implies that while it may have overlapping roles as HO-1, it may have distinctive functions that are as yet unknown or unassociated with cirrhosis.
NO has been found to stabilize HO-1 mRNA as well induce HO-1 in a cGMP-independent fashion[19]. The increase of HO may be in part due to NO from splanchnic circulation or from the shear stress faced within the liver. These serve to provide CO, a vasodilator, in the absence of adequate amounts of NO from eNOS or iNOS. Increased production of vasodilator CO in Kupffer cells possibly aids the alleviation of shear stress. CO, unlike NO, is not a radical and is known to be a thousand-fold less potent than NO with respect to vasodilation and activation of cGMP[16]. CO signaling may be particularly pertinent in conditions of oxidative stress[17-20]. Exhaled CO has been found to be significantly increased in cirrhotic patients in comparison to healthy individuals[20]. At low NO levels, such as in cirrhotic livers, CO can act a vasodilator through stimulation of soluble guanylate cyclase[20].Taken together, HO and its product CO may act to counteract portal hypertension in cirrhosis.
In summary, we demonstrated major differences between the expression and localization of eNOS and iNOS. Possibly these differences allude to their differing roles in maintaining liver homeostasis or involvement in the pathology of cirrhosis. The lack of difference in iNOS expression between cirrhotic and non-cirrhotic tissue suggests iNOS involvement in the pathology of other liver diseases as truly normal liver tissues were not available. The increased expression of caveolin-1 within the cirrhotic liver is also suggestive of some form of regulation imposed upon eNOS. Whether this serves as a defense mechanism against further cirrhosis or as a result of cirrhosis with escalating portal hypertension as a consequence, is yet unknown. HO-1 and HO-2 are differentially expressed. It is possible that CO and NO have parallel or coordinated functions within the liver and may work together in the pathophysiology of portal hypertension.
S- Editor Kumar M, Wang J and Guo SY L- Editor Elsevier HK E- Editor Bi L
| 1. | Rockey DC. Vascular mediators in the injured liver. Hepatology. 2003;37:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Desmet VJ, Roskams T. Cirrhosis reversal: a duel between dogma and myth. J Hepatol. 2004;40:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 168] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Hon WM, Lee KH, Khoo HE. Nitric oxide in liver diseases: friend, foe, or just passerby. Ann N Y Acad Sci. 2002;962:275-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Arroyo V, Jimenez W. Complications of cirrhosis. II. Renal and circulatory dysfunction. Lights and shadows in an important clinical problem. J Hepatol. 2000;32:57-170. |
| 5. | Zakhary R, Poss KD, Jaffrey SR, Ferris CD, Tonegawa S, Snyder SH. Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc Natl Acad Sci USA. 1997;94:14848-14853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 184] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Farzaneh-Far R, Moore K. Nitric oxide and the liver. Liver. 2001;21:161-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Clemens MG. Nitric oxide in liver injury. Hepatology. 1999;30:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 133] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Wiest R, Groszmann RJ. The paradox of nitric oxide in cirrhosis and portal hypertension: too much, not enough. Hepatology. 2002;35:478-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 308] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 9. | Saetre T, Gundersen Y, Thiemermann C, Lilleaasen P, Aasen AO. Aminoethyl-isothiourea, a selective inhibitor of inducible nitric oxide synthase activity, improves liver circulation and oxygen metabolism in a porcine model of endotoxemia. Shock. 1998;9:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | McNaughton L, Puttagunta L, Martinez-Cuesta MA, Kneteman N, Mayers I, Moqbel R, Hamid Q, Radomski MW. Distribution of nitric oxide synthase in normal and cirrhotic human liver. Proc Natl Acad Sci USA. 2002;99:17161-17166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Mohammed NA, Abd El-Aleem S, Appleton I, Maklouf MM, Said M, McMahon RF. Expression of nitric oxide synthase isoforms in human liver cirrhosis. J Pathol. 2003;200:647-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Wei CL, Khoo HE, Lee KH, Hon WM. Differential expression and localization of nitric oxide synthases in cirrhotic livers of bile duct-ligated rats. Nitric Oxide. 2002;7:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Sarela AI, Mihaimeed FM, Batten JJ, Davidson BR, Mathie RT. Hepatic and splanchnic nitric oxide activity in patients with cirrhosis. Gut. 1999;44:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Feron O, Saldana F, Michel JB, Michel T. The endothelial nitric-oxide synthase-caveolin regulatory cycle. J Biol Chem. 1998;273:3125-3128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 283] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Yokomori H, Oda M, Yoshimura K, Nomura M, Wakabayashi G, Kitajima M, Ishii H. Elevated expression of caveolin-1 at protein and mRNA level in human cirrhotic liver: relation with nitric oxide. J Gastroenterol. 2003;38:854-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Wei CL, Lee KH, Khoo HE, Hon WM. Expression of haem oxygenase in cirrhotic rat liver. J Pathol. 2003;199:324-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Makino N, Suematsu M, Sugiura Y, Morikawa H, Shiomi S, Goda N, Sano T, Nimura Y, Sugimachi K, Ishimura Y. Altered expression of heme oxygenase-1 in the livers of patients with portal hypertensive diseases. Hepatology. 2001;33:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Sacerdoti D, Abraham NG, Oyekan AO, Yang L, Gatta A, McGiff JC. Role of the heme oxygenases in abnormalities of the mesenteric circulation in cirrhotic rats. J Pharmacol Exp Ther. 2004;308:636-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Ryter SW, Otterbein LE, Morse D, Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem. 2002;234-235:249-263. [PubMed] |
| 20. | De las Heras D, Fernández J, Ginès P, Cárdenas A, Ortega R, Navasa M, Barberá JA, Calahorra B, Guevara M, Bataller R. Increased carbon monoxide production in patients with cirrhosis with and without spontaneous bacterial peritonitis. Hepatology. 2003;38:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |