Published online Jan 28, 2006. doi: 10.3748/wjg.v12.i4.516
Revised: June 30, 2005
Accepted: July 15, 2005
Published online: January 28, 2006
Massive ascites and hepatorenal syndrome (HRS) are frequent complications of liver cirrhosis. Thus, effective therapy is of great clinical importance. This concise review provides an update of recent advances and new developments. Therapeutic paracentesis can be safely performed even in patients with severe coagulopathy. Selected patients with a refractory or recurrent ascites are good candidates for non-surgical portosystemic shunts (TIPS) and may have a survival benefit and improvement of quality of life. Novel pharmaceutical agents mobilizing free water (aquaretics) are currently under test for the therapeutic potential in patients with ascites.
Prophylaxis of hepatorenal syndrome in patients with spontaneous bacterial peritonitis is recommended and should be considered in patients with alcoholic hepatitis. Liver transplantation is the best therapeutic option with long-term survival benefit for patients with HRS. To bridge the time until transplantation, TIPS or Terlipressin and albumin are good options. Albumin dialysis can not be recommended outside prospective trials.
- Citation: Gerbes AL, Gulberg V. Progress in treatment of massive ascites and hepatorenal syndrome. World J Gastroenterol 2006; 12(4): 516-519
- URL: https://www.wjgnet.com/1007-9327/full/v12/i4/516.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i4.516
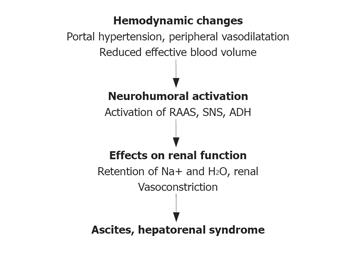
Haemodynamic alterations and activation of neurohumoral systems are essential in the pathophysiology of ascites formation[1,2]. The most common circulatory alterations in patients with cirrhosis of the liver are portal hypertension and peripheral arterial vasodilatation which results in a decrease of centrally effective blood volume. As a consequence, neurohumoral systems are activated in an attempt to maintain intravascular volume (Figure 1). Among those, activation of the renin-angiotensin-aldosterone system, the sympathetic nervous system and the non-osmotic release of arginin-vasopressin play the major role. These neurohumoral systems induce renal sodium and water retention leading to the formation of ascites. The most severe form with renal vasoconstriction and a decrease of renal blood flow leads to hepatorenal syndrome. Recently, it has been suggested that a decrease in cardiac output may contribute to HRS[3].
Novel therapeutic strategies for ascites and hepatorenal syndrome thus focus to counteract the initial changes of this pathophysiological cascade: controlled reduction of portal hypertension and/or peripheral vasoconstriction combined with plasma expanders.
In order to achieve fast relief, patients with massive ascites undergo therapeutic paracentesis[4]. Apart from the risk of infection which can easily be prevented, major concerns have been raised regarding the risk of bleeding and haemodynamic instability. It has been shown that large volume paracentesis can be safely performed even in patients with severely impaired coagulation parameters[5,6]. In a study investigating more than 500 patients with cirrhosis of the liver, an average paracentesis of 9 g/L was performed[5]. Although the majority of patients exhibited less than 50 × 109/L platelets and more than a quarter had severely prolonged prothrombin time, no case had severe complication or bleeding.
Following large volume paracentesis rapid re-formation of ascites may effectively reduce central blood volume and compromise systemic haemodynamic and renal function. To prevent intravenous administration of plasma expanders, a paracentesis has been established[2,7]. For large volume paracentesis (more than 6 L) 20% human albumin in a concentration of at least 6 g per liter ascites seems to be the safest strategy. Recent work demonstrated that the incidence of paracentesis-induced circulatory disfunction following paracentesis of less than 6 L is only 7% with albumin or saline as plasma expanders with almost no clinical complications[8]. Thus, it may be safe and cost-effective to perform paracentesis of up to 6 L without albumin.
Refractory and recurrent ascites have been defined according to a consensus conference of the International Ascites Club[9] (Table 1). Repeated large volume paracentesis in addition to diuretic treatment is the standard treatment for these patients. Reduction of portal hypertension with non-surgical shunts (transjugular intrahepatic portosystemic shunt = TIPS) has the potential to markedly increase renal sodium excretion[10]. This has prompted several prospective randomized controlled trials comparing repeated paracentesis with insertion of a TIPS (Table 2). The unequivocal result of all four trials[11-14] has a highly significant advantage in the control of ascites by TIPS. Regarding survival, two trials have shown a significant advantage. Possibly, inclusion of patients with more severely impaired liver function and a bilirubin above 5mg/100mL at inclusion may be responsible for the lack of survival benefit following TIPS[15] (Table 3). Encephalopathy following TIPS should be avoided as the major obstacle for improvement of quality of life as compared to paracentesis patients[13]. Thus, careful selection of patients and management in experienced centers are the prerequisite for a TIPS benefit. Patients with ascites resolution following TIPS insertion enjoy a markedly improved quality of life[16].
| Refractory ascites | cannot be mobilized by diuretics because of a lack of response (mean weight loss less than 200g/d during the last 4 d) or the development of diuretic-induced complications such as hyponatremia, hypokalemia, renal impairment, hepatic encephalopathy, precluding an effective diuretic dosage |
| Recidivant ascites | recurs at least on 3 occasions within 1 year despite prescription of dietary sodium restriction and adequate diuretic dosage |
| Rossle[11] | Gines[12] | Sanyal[13] | Salerno[14] | |
| Patients/selected from pts. | 60/155 | 70/119 | 109/525 | 66/137 |
| Complete response (%) | 79 vs 24 | 51 vs 17 | 58 vs 16 | 61 vs 3 |
| Survival benefit of TIPS | yes | no | trend | yes |
| Number of centers | 2 | ≥5 | 6 | 3 |
| Child-Pugh C (%) | 38 | 37 | ? | 76 |
| Athyltox. Zirrhose (%) | 79 | 51 | 62 | 42 |
| Severe encephalop. (%) | 23 vs 13 | 60 vs 34 | 29 vs 18 | 61 vs 39 |
| Mean TIPS Ø (mm) | 9 | 8→10 | 10 | ? |
Vasopressin-V2-receptor antagonists mobilize free water and thus might be an excellent alternative or adjunct to diuretic treatment. The efficacy and safety of these new compounds in patients with cirrhosis have recently been demonstrated[17,18]. In these patients, urine volume increased and urine osmolarity dose-dependently decreased, thus demonstrating an increase of free water clearance. Moreover, while patients in the placebo group gained weight, body weight was stable with a lower dose and clearly decreased with a higher dose of the V2-receptor antagonist. This promising new pharmaceutical concept is currently under investigation in international phase II/III trials.
According to established criteria, hepatorenal syndrome can be classified into type 1 and type 2[9] (Table 4). Rapid progressive type 1 exhibits a very poor prognosis with a 3-month mortality rate of above 90%[19]. Thus, prophylaxis of HRS is an important task and effective treatment is a highly desirable goal.
| HRS Type 1: Rapidly progressing renal failure (< 2 wk) ≥ 2-fold increase of serum creatinine to > 221μmol/L or 50% decrease of creatinine clearance to < 20mL/min |
| HRS Type 2: Not rapidly progressing renal failure |
| Serum creatinine > 132.6 μmol/L or |
| Creatinine clearance < 40mL/min |
| Absence of shock, ongoing bacterial infection, current or recent treatment with nephrotoxic drugs, gastrointestinal or renal fluid loss |
| No sustained improvement upon withdrawal of diuretics and plasma volume expansion |
| Proteinuria < 0.5g/d, no abnormalities of renal ultrasound |
In patients with severe alcoholic hepatitis, the TNF-α inhibitor pentoxyfillin significantly reduces the incidence of HRS, HRS-related and over-all mortality[20]. Spontaneous bacterial peritonitis is often followed by deterioration of renal function and even hepatorenal syndrome. Interestingly, a randomized prospective trial[21] showed that intravenous albumin administration (1.5g/kg per day on day one and 1g/kg per day on day three) together with antibiotic treatment with cefotaxim is clearly superior to antibiotic treatment alone because renal failure and moreover mortality were significantly reduced in hospital and during a 3-month follow-up period.
Following liver transplantation, patients with HRS may reach 5-year survival probability of 60%[22]. While this is a tremendous improvement compared to the spontaneous prognosis, survival rates are significantly lower than those in patients undergoing liver transplantation with normal renal function. Moreover, in many Western countries, waiting lists for liver transplantation are steadily growing, thus increasing the need to bridge severely sick patients to transplantation. Therapeutic concepts are needed to normalize renal function in patients with hepatorenal syndrome type 1. As stated in the introduction, very early interventions in the pathomechanistic cascade would seem more promising.
Indeed, controlled reduction of portal hypertension with TIPS seems to be rather effective. In an uncontrolled trial, the average mean survival time was around 4 mo in 14 out of 23 patients with HRS type 1 who received TIPS[23]. Nine of the 23 patients, however, were at high risk for liver failure and therefore did not receive TIPS. For these severely ill patients, strategies should be used to counteract peripheral vasodilatation combined with volume expansion.
Several studies suggest that vasopressin analogues combined with albumin may be suitable to reverse hepatorenal syndrome[24-26]. Terlipressin (average daily dose of 3 mg) could be a valuable option for patients with HRS type 1 and very poor liver function to bridge the time to transplantation if combined with plasma expanders[27]. In this retrospective analysis best results with terlipressin were seen in patients with Child-Pugh score below 12 points, receiving at least 3 mg per day. However, only one randomized controlled trial on terlipressin has shown improvement of renal function in patients with HRS type 1 compared to no response in the placebo group[28]. No survival data are provided.
In numerous uncontrolled observations extracorporeal albumin dialysis (MARS) has been suggested as a beneficial therapy for patients with acute-on-chronic liver failure. There are only two randomized trials investigating the effects of MARS, comprising a total of 23 patients with hepatorenal syndrome[29,30]. No 30-d survival benefit has been demonstrated, disqualifying this procedure for the use outside of controlled trials[30].
The assistance of Anne Esmark in preparing and editing the manuscript is greatly appreciated.
S- Editor Wang XL, Pan BR and Guo SY L- Editor Elsevier HK E- Editor Bi L
| 1. | Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1131] [Cited by in RCA: 1022] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 2. | Gerbes AL. [Experimental methods in hepatology. Guidelines of the German Work Group for Study of the Liver. Therapy of ascites in liver diseases. German Work Group for Study of the Liver]. Z Gastroenterol. 1997;35:295-300. [PubMed] |
| 3. | Ruiz-del-Arbol L, Monescillo A, Arocena C, Valer P, Ginès P, Moreira V, Milicua JM, Jiménez W, Arroyo V. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42:439-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 383] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 4. | Runyon BA. Management of adult patients with ascites due to cirrhosis. Hepatology. 2004;39:841-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Grabau CM, Crago SF, Hoff LK, Simon JA, Melton CA, Ott BJ, Kamath PS. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40:484-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Lin CH, Chen SC, Ko PC. Preprocedure coagulation tests are unnecessary before abdominal paracentesis in emergency departments. Hepatology. 2005;41:402-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, Angeli P, Porayko M, Moreau R, Garcia-Tsao G. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 611] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 8. | Sola-Vera J, Miñana J, Ricart E, Planella M, González B, Torras X, Rodríguez J, Such J, Pascual S, Soriano G. Randomized trial comparing albumin and saline in the prevention of paracentesis-induced circulatory dysfunction in cirrhotic patients with ascites. Hepatology. 2003;37:1147-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, Reynolds TB, Ring-Larsen H, Schölmerich J. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1020] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 10. | Gerbes AL, Gülberg V, Waggershauser T, Holl J, Reiser M. Renal effects of transjugular intrahepatic portosystemic shunt in cirrhosis: comparison of patients with ascites, with refractory ascites, or without ascites. Hepatology. 1998;28:683-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Rössle M, Ochs A, Gülberg V, Siegerstetter V, Holl J, Deibert P, Olschewski M, Reiser M, Gerbes AL. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med. 2000;342:1701-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 380] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 12. | Ginès P, Uriz J, Calahorra B, Garcia-Tsao G, Kamath PS, Del Arbol LR, Planas R, Bosch J, Arroyo V, Rodés J. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology. 2002;123:1839-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 364] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 13. | Sanyal AJ, Genning C, Reddy KR, Wong F, Kowdley KV, Benner K, McCashland T. The North American Study for the Treatment of Refractory Ascites. Gastroenterology. 2003;124:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 282] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | Salerno F, Merli M, Riggio O, Cazzaniga M, Valeriano V, Pozzi M, Nicolini A, Salvatori F. Randomized controlled study of TIPS versus paracentesis plus albumin in cirrhosis with severe ascites. Hepatology. 2004;40:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 236] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 15. | Gerbes AL, Gülberg V. Benefit of TIPS for patients with refractory or recidivant ascites: serum bilirubin may make the difference. Hepatology. 2005;41:217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Gülberg V, Liss I, Bilzer M, Waggershauser T, Reiser M, Gerbes AL. Improved quality of life in patients with refractory or recidivant ascites after insertion of transjugular intrahepatic portosystemic shunts. Digestion. 2002;66:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Gerbes AL, Gülberg V, Ginès P, Decaux G, Gross P, Gandjini H, Djian J. Therapy of hyponatremia in cirrhosis with a vasopressin receptor antagonist: a randomized double-blind multicenter trial. Gastroenterology. 2003;124:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 190] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Wong F, Blei AT, Blendis LM, Thuluvath PJ. A vasopressin receptor antagonist (VPA-985) improves serum sodium concentration in patients with hyponatremia: a multicenter, randomized, placebo-controlled trial. Hepatology. 2003;37:182-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 179] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 19. | Ginès P, Guevara M, Arroyo V, Rodés J. Hepatorenal syndrome. Lancet. 2003;362:1819-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 372] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 20. | Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 507] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 21. | Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1003] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 22. | Gonwa TA, Klintmalm GB, Levy M, Jennings LS, Goldstein RM, Husberg BS. Impact of pretransplant renal function on survival after liver transplantation. Transplantation. 1995;59:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 280] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Brensing KA, Textor J, Perz J, Schiedermaier P, Raab P, Strunk H, Klehr HU, Kramer HJ, Spengler U, Schild H. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non-transplant cirrhotics with hepatorenal syndrome: a phase II study. Gut. 2000;47:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 286] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 24. | Gülberg V, Bilzer M, Gerbes AL. Long-term therapy and retreatment of hepatorenal syndrome type 1 with ornipressin and dopamine. Hepatology. 1999;30:870-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 116] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Uriz J, Ginès P, Cárdenas A, Sort P, Jiménez W, Salmerón JM, Bataller R, Mas A, Navasa M, Arroyo V. Terlipressin plus albumin infusion: an effective and safe therapy of hepatorenal syndrome. J Hepatol. 2000;33:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 246] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 26. | Wong F, Blendis L. New challenge of hepatorenal syndrome: prevention and treatment. Hepatology. 2001;34:1242-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Moreau R, Durand F, Poynard T, Duhamel C, Cervoni JP, Ichaï P, Abergel A, Halimi C, Pauwels M, Bronowicki JP. Terlipressin in patients with cirrhosis and type 1 hepatorenal syndrome: a retrospective multicenter study. Gastroenterology. 2002;122:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 275] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 28. | Solanki P, Chawla A, Garg R, Gupta R, Jain M, Sarin SK. Beneficial effects of terlipressin in hepatorenal syndrome: a prospective, randomized placebo-controlled clinical trial. J Gastroenterol Hepatol. 2003;18:152-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 188] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 29. | Mitzner SR, Stange J, Klammt S, Risler T, Erley CM, Bader BD, Berger ED, Lauchart W, Peszynski P, Freytag J. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transpl. 2000;6:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 401] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 30. | Sen S, Davies NA, Mookerjee RP, Cheshire LM, Hodges SJ, Williams R, Jalan R. Pathophysiological effects of albumin dialysis in acute-on-chronic liver failure: a randomized controlled study. Liver Transpl. 2004;10:1109-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |