Published online Oct 14, 2006. doi: 10.3748/wjg.v12.i38.6239
Revised: July 8, 2006
Accepted: July 20, 2006
Published online: October 14, 2006
We present ultrasound, computed tomography and magnetic resonance imaging findings in a case with pancreatic solid pseudopapillary tumor and their correlations with histopathology. Ultrasound revealed a hypoechogenic mass, and computed tomography revealed a hypodense mass at the pancreatic head minimally enhanced after intravenous contrast agent administration. Magnetic resonance imaging showed a hypointense mass on unenhanced T1-weighted images including a hyperintense focus representing the hemorrhage. The lesion was hyperintense on T2-weighted images. On the postcontrast images the lesion showed peripheral thin contrast enhancement in arterial phase and enhanced slightly diffusely in venous and equilibrium phases. The patient underwent elective resection of the mass and pancreatoduodenectomy with jejunostomy tube placement. A final diagnosis of solid pseudopapillary tumor was made histopathologically. Solid pseudopapillary tumor is a rare pancreatic tumor. It is important to make the diagnosis preoperatively because with an adequate surgical resection the prognosis is good. A multimodalitary approach, especially magnetic resonance imaging can suggest the diagnosis without the need for biopsy.
- Citation: Karatag O, Yenice G, Ozkurt H, Basak M, Basaran C, Yilmaz B. A case of solid pseudopapillary tumor of the pancreas. World J Gastroenterol 2006; 12(38): 6239-6243
- URL: https://www.wjgnet.com/1007-9327/full/v12/i38/6239.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i38.6239
Most pancreatic tumors are malignant and have a bad prognosis. However, solid-pseudopapillary tumor of the pancreas (SPT) is a rare benign or low-grade malignancy. Because of this, it is important to make the diagnosis of this tumor preoperatively so that adequate resection will be undertaken. SPT accounts for less than 1%-2% of the exocrine pancreatic tumors[1-5]. It affects mostly young females between the 2nd and 3rd decades of life with a male to female ratio of 1:9. The patients with SPT are often clinically asymptomatic or minimally symptomatic[3,6,7]. Therefore imaging studies should be carefully assessed[1,2]. Ultrasonography (US), computed tomography (CT) and magnetic resonance (MR) imaging are the useful modalities in the diagnosis of SPT which is not uncommonly diagnosed incidentally at abdominal imaging[7]. Here we report a case of a 17-year old girl presenting with this rare pancreatic tumor and discuss the differential diagnosis from other pancreatic masses.
A 17-year old woman complaining of dyspeptic symptoms and right upper quadrant pain for several months was admitted to our hospital for further evaluation. She had no history of abdominal trauma or surgery, drug usage or smoking. On physical examination tenderness in the right upper quadrant and epigastrium was noted. Laboratory findings showed no abnormalities.
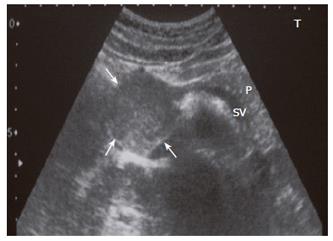
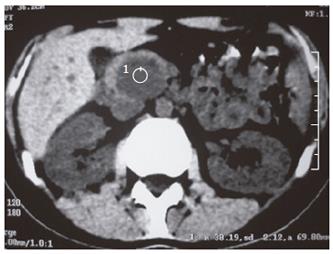
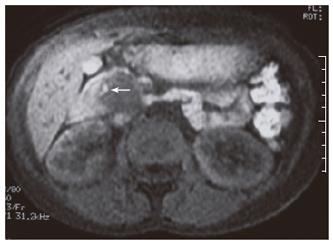
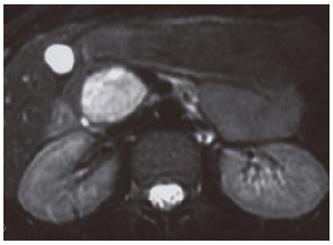
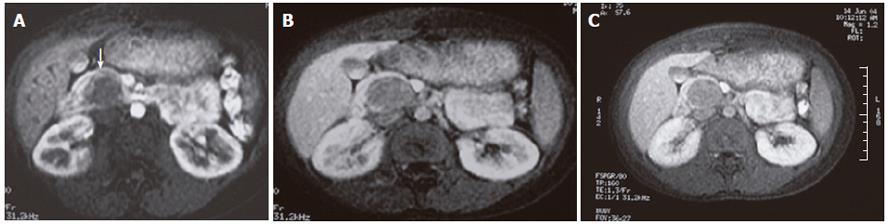
An abdominopelvic US revealed a heterogenous hypoechogenic solid mass lesion at the pancreatic head region (Figure 1). CT scan showed a round shaped, well-marginated, hypodense mass at the pancreatic head with a density of 38.19 HU and no evidence of internal hemorrhage (Figure 2). After intravenous iodine contrast agent administration, the lesion showed minimally heterogenous contrast enhancement markedly less than the normal pancreatic tissue. MR examination included axial fast spoiled gradient-echo (FSPGR) fat saturated T1-weighted images and axial and coronal single-shot fast spin-echo T2-weighted images. Unenhanced and contrast-enhanced arterial, portal venous and equilibrium phase images were obtained. MR showed that the mass lesion was predominantly hypointense on unenhanced T1-weighted images including a hyperintense focus representing the hemorrhagic degeneration (Figure 3). The lesion was predominantly hyperintense (but less than cerebrospinal fluid) on T2-weighted images mostly composed of solid and minimally cystic areas (Figure 4). On the initial arterial-phase contrast-enhanced images the lesion was predominantly hypointense with some peripheral thin contrast enhancement (Figure 5A). On the portal venous and equilibrium phase images the lesion seemed to enhance diffusely slightly (Figure 5B and 5C).
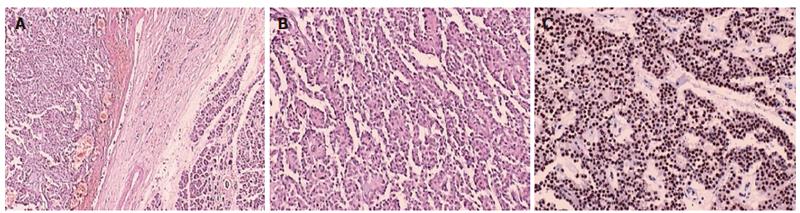
The patient underwent elective resection of the mass in the pancreatic head and pancreaticoduodenectomy (Whipple procedure) with jejunostomy tube placement. The resection material was sent to the pathology department. At gross examination, a well-circumscribed, capsulated, round, gray-purple colored tumor was identified in the pancreatic head. By histologic analysis, pseudopapillary aggregates including stromal central vessels were detected. They were separated with partially defective thin fibrous capsule from the pancreatic tissue leading to some pancreatic invasion locally. The tumor was composed of uniform polygonal cells with prominent nucleolus, uniform nucleus and narrow eosinophilic cytoplasms (Figure 6A and 6B). No vascular space or perineural invasion was identified. By immunohistochemical analysis, the tumor cells were positive for vimentin and synaptophysin, and for progesterone receptors (Figure 6C); and negative for cytokeratin, chromogranin and estrogen receptors. In the evaluation of Ki-67, less than 5% of the tumor cells showed nuclear proliferative activity. These are compatible histopathologic findings for SPTs except for synaptophysin positivity which is unusual.
After the surgery the patient underwent MR imaging examination per 6 mo for the first year and then once a year for the following 2 years until the present and no recurrence, metastases or symptoms occured.
SPT is a rare pancreatic tumor consisting of 1%-2% of exocrine pancreatic tumors[1,7]. The precise incidence of SPT is not known because it is rare and is frequently misdiagnosed[1]. This tumor was first described by Frantz in 1959 as a “papillary tumor of the pancreas, benign or malignant”[8]. Since then, various names have been used to describe this rare lesion, such as solid and cystic tumor of the pancreas, papillary-cystic tumor, solid and papillary epithelial neoplasm and Frantz tumor[1,2,7]. In 1996, the World Health Organization (WHO) renamed this tumor as SPT for the international histologic classification of tumor of the exocrine pancreas[7,9].
SPTs are benign lesions but sometimes malignant degeneration may occur especially when the lesion occurs in elderly male patients[2,7].
SPT of the pancreas has distinctive pathologic features. The mass is most frequently found in the head or tail region. On gross examination, the mass is usually large and well encapsulated and contains varying amounts of necrosis, hemorrhage, and cystic change. By microscopic analysis, there are two distinct types of cellular arrangements: solid and papillary[7]. The pathogenesis of these tumors is still controversial. Whereas some authors postulate an endocrine origin, others claim these tumors arise from ductal cells, acinar cells, or pluripotent stem cells[1]. Vito et al[1] claimed that, SPT was associated with pregnancy in 3, with polycystic ovary disease in 2, with Sertoli-Leydig cell tumor in 1 of their 19 patients, which supports an endocrine origin for SPT of pancreas[1]. Also a hormonal influence is also suggested in view of the tumor’s high prevalence in women.
SPTs are typically positive for vimentin, neuron-specific enolase (NSE), α-1-antitrypsin, and α-1-antichymotrypsin and negative for chromogranin A, epithelial membrane antigen, and cytokeratin. Differentiation along endocrine cell lines has been postulated for this tumor on the basis of NSE positivity, but the expression of vimentin and α-1-antitrypsin does not support this interpretation[7]. A study by Kosmahl et al[10] demonstrated that SPTs have a complex immunoprofile that is inconsistent with that of any of the pancreatic cell types and that a pancreatic origin is unlikely. The authors speculated that on the basis of some similarities between SPT and ovarian surface cells and the proximity between genital ridges and the pancreas anlage during early embryogenesis, SPTs might originate from the genital ridge-related cells that were incorporated into the pancreas during organogenesis. Furthermore, sex hormones may play a role in the pathogenesis or growth of SPTs: Nearly all studies demonstrate no evidence of estrogen receptors; however, progesterone receptors are present in many cases[7,11] as presented in our case.
The origin of SPTs has not yet been clarified. SPTs cannot be regarded as a purely endocrine neoplasm because the presence of neuroendocrine markers is not significant (chromogranin A is not detected, synaptophysin has a patchy immunoreactivity in 22% and NSE is strongly positive in more than 90% but without a certain significance)[12-14].
However, SPTs are classified and are generally held to be epithelial neoplasms but immunohistochemical patterns suggest that SPTs cannot be regarded as purely epithelial neoplasms[12,15]. In fact, cytokeratin expression, usually associated with epithelial differentiation, is rare (less than 30%) or absent in most cases, and markers of acinar differentiaton (trypsin/chymotrypsin) and glycoprotein markers of ductal differentiation are often negative[12,16,17].
Although all these hypotheses of origin are intriguing, they are yet unproved, and further research is necessary.
Another interesting point is the relationship between the synaptophysin positivity and recurrence rates of SPTs. Lai et al[18] analysed 7 patients histopathologically and found that 6 of the patients with no recurrence were negative for synaptophysin and 1 of the patients who suffered from recurrence was positive. In our case synaptophysin was unusually positive with no recurrence after surgical management in the period of 3 years until now.
Patients with SPT are often clinically asymptomatic[2,3,6,7]. They may present with palpable abdominal mass, abdominal pain or discomfort[1-3,6,7] and also acute abdomen can develop due to the rupture of the lesion[2,3,19]. The abdomen is usually nontender on palpation but obstructive symptoms may occur if the tumor grows large enough to compress adjacent anatomic structures[7]. However, bile duct or pancreatic duct obstruction is rare, even when it is located in the head of the pancreas because of its softness[1]. Physical examination is often normal apart from the presence of an upper abdominal mass[2], and usually no laboratory abnormalities appear associated with pancreatic insufficiency, abnormal liver funcion, cholestasis, elevated pancreatic enzymes, or an endocrine syndrome. Tumor markers are also expected to be normal[2,7]. Also an unusual presentation of SPT with acute pancreatitis was reported in the literature[6].
Given the good prognosis of the disease, it is important to make the diagnosis preoperatively so that adequate resection will be undertaken. Therefore imaging studies should be carefully assessed[1,2]. US, CT and MR are the useful diagnostic modalities in the diagnosis of SPT which is not uncommonly diagnosed incidentally at abdominal imaging [7].
On US examination the lesion is well-encapsulated and includes cystic and solid components, but sometimes the mass is pure and solid-looking or has internal septa or calcifications[7]. Usually the appearance of SPT is variable and lacks correlation with gross pathology[1].
Contrast-enhanced CT plays a major role in the diagnostic evaluation of cystic neoplasms of the pancreas. However, when compared with MR imaging, CT has inherent limitations in showing certain tissue characteristics, such as hemorrhage, cystic degeneration, or the presence of a capsule. These features may, as shown at pathology, be suggestive of specific lesions such as SPT of the pancreas. Therefore, MR imaging may further aid in showing these characteristics and in the differential diagnosis of complex cystic masses within the pancreas[1].
CT usually demonstrates a well-encapsulated, heterogenous lesion with varying solid and cystic components[7]. After intravenous contrast agent administration, enhanced solid areas are typically noted peripherally, whereas cystic spaces are usually more centrally located[7].
MR imaging, because of its superior contrast resolution, displays a capsule and intratumoral hemorrhage better than CT. Therefore, MR imaging has the potential to improve our ability to diagnose SPT[1]. MR images demonstrate a well-defined lesion with heterogenous signal intensity on T1 and T2-weighted images, which reflects the complex nature of the mass[7]. In previous reports all SPTs contain some high signal intensity on T1-weighted images representing the blood products[1,7]. A hyperintense focus was also noted on T1-weighted axial images of our case. Vito et al[1] also defined that 5 of 19 patients did not show high signal on T1-weighted images in their study.Therefore, the absence of high T1 signal should not exclude the diagnosis.
The T2-weighted appearance was described differently in the literature. Ohtomo et al[20] reported findings for six patients: the masses appeared hyperintense in four patients and mixed-that is, hyper- and hypointense-in two patients. Buetow et al[21] reported areas of high signal intensity on T2-weighted images in nine patients and areas of low signal intensity in three patients. However, they did not report the predominant signal intensity of the masses or whether the lesions appeared homogeneous. Vito et al[1] reported findings for 19 patients: 14 lesions (73.5%) appeared heterogeneous, and all lesions but two (89%) appeared predominantly hyperintense on T2-weighted images. In our case the lesion was also predominantly hyperintense on T2-weighted images.
Angiography usually demonstrates an avascular or hypovascular pancreatic tumor and may help delineate the mass from other involved and adjacent structures[2]. Vito et al[1] reported that most common enhancement pattern of SPT consists of early, peripheral, and heterogeneous enhancement during the arterial phase with progressive but heterogeneous fill-in of the lesion during the portal venous and equilibrium phases. They also reported that all but one lesion in their series enhanced less than the adjacent normal pancreas during all phases. This feature helps distinguishing SPT from other pancreatic neoplasms, such as islet cell tumors, which typically enhance more than the pancreas. Vito et al[1] reported that all but one of their patients had a peripheral hypointense tumor capsule on both T1- and T2-weighted images. Compared with the lesion, the tumor capsule showed early and more intense enhancement (70%), identical enhancement (20%), or less enhancement (10%). Ohtomo et al[20] reported that four patients (67%) presented with a fibrous capsule detected as a band of low signal intensity on T1- and T2-weighted imaging. Conversely, Buetow et al[21] observed in all their patients a discontinuous low-signal-intensity rim on T2-weighted images. In our case a capsule formation was not detected.
Serous microcystic adenoma, mucinous cystic neoplasm, cystic islet cell tumor, pancreaticoblastoma, and calcified hemorrhagic pseudocyst are differential diagnostic considerations when a pancreatic mass consists of cystic and solid components. The former three tumors occur rarely in patients younger than 30 years[1,22]. On T1-weighted MR images, serous microcystic adenoma may appear as a heterogeneous hypointense mass with hyperintense foci related to prior hemorrhage and could be confused with SPT. However, on T2-weighted images, serous microcystic adenoma shows hyperintense signal intensity with low-signal-intensity central areas due to scar formation and hypointense septa that may radiate toward an enhanced central scar[1,22,23]. This radial central scar is not a feature of SPT[1].
Mucinous cystic neoplasms may be confused with SPT, particularly when the SPT is predominantly cystic[1,22,23]. Although the mucin-filled cystic spaces are typically hyperintense on T2-weighted images and hypointense on T1-weighted images, mucin occasionally results in high signal intensity on both T1- and T2-weighted images[1,23]. The signal intensity may vary depending on the proteinaceous content of the mucin. Also, mucinous cystic neoplasms do not exhibit early peripheral and capsular enhancement[1].
Islet cell tumors occur in patients who are older and do not have the female predominance. They may appear cystic, contain calcifications, and show areas of internal hemorrhage[1,21,24]. Islet cell tumors are low in signal intensity on fat-suppressed T1-weighted images, exhibit high signal intensity on T2-weighted images, and show marked ring or diffuse heterogeneous enhancement on immediate gadolinium-enhanced gradient-echo images[1,21]. The differential diagnosis of SPT from islet cell tumor of the pancreas is the different signal intensity on T1-weighted images and excess contrast enhancement of islet cell tumors after gadolinium injection due to their more vascularity compared with SPTs[1].
Pancreatoblastoma is more aggressive than SPT and often presents with liver metastases at the time of diagnosis. Intratumoral hemorrhage has not been reported in these tumors using either CT or MR imaging. Although pancreatic adenocarcinoma is the most common primary pancreatic malignancy, it is seen in older patients. Pancreatic pseudocysts may be calcified peripherally as a result of internal hemorrhage and may mimic SPT. However, a history of pancreatitis is almost always present, and pancreatic pseudocysts can be distinguished from SPT by the absence of solid components[1].
In conclusion, when a young woman presents with a pancreatic mass lesion, SPT must be considered in the differential diagnosis. Multimodalitary approach, especially MR could suggest the diagnosis of SPT with some imaging features including well-marginated, encapsulated, solid-cystic mass with areas of hemorrhagic degeneration and progressive peripheral or heterogenous contrast enhancement.
S- Editor Pan BR L- Editor Zhu LH E- Editor Bi L
| 1. | Cantisani V, Mortele KJ, Levy A, Glickman JN, Ricci P, Passariello R, Ros PR, Silverman SG. MR imaging features of solid pseudopapillary tumor of the pancreas in adult and pediatric patients. AJR Am J Roentgenol. 2003;181:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 162] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 2. | Huang HL, Shih SC, Chang WH, Wang TE, Chen MJ, Chan YJ. Solid-pseudopapillary tumor of the pancreas: clinical experience and literature review. World J Gastroenterol. 2005;11:1403-1409. [PubMed] |
| 3. | Meshikhes AW, Atassi R. Pancreatic pseudopapillary tumor in a male child. JOP. 2004;5:505-511. [PubMed] |
| 4. | Fernández-del Castillo C, Warshaw AL. Cystic tumors of the pancreas. Surg Clin North Am. 1995;75:1001-1016. [PubMed] |
| 5. | Dong PR, Lu DS, Degregario F, Fell SC, Au A, Kadell BM. Solid and papillary neoplasm of the pancreas: radiological-pathological study of five cases and review of the literature. Clin Radiol. 1996;51:702-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Sakagami J, Kataoka K, Sogame Y, Taii A, Ojima T, Kanemitsu D, Takada R, Ito R, Motoyoshi T, Yasuda H. Solid pseudopapillary tumor as a possible cause of acute pancreatitis. JOP. 2004;5:348-352. [PubMed] |
| 7. | Coleman KM, Doherty MC, Bigler SA. Solid-pseudopapillary tumor of the pancreas. Radiographics. 2003;23:1644-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Frantz VK. Tumors of the pancreas. Atlas of Tumor Pathology, Section VII, fasc. 27 and 28. Washington, DC: Armed Forces Institute of Pathology 1959; 32-33. |
| 9. | Kloppel G, Solcia E, Longnecker DS, Capella C, Sobin LH. Histological typing of tumours of the exocrine pancreas. Berlin: Springer-Verlag 1996; 11-20. [DOI] [Full Text] |
| 10. | Kosmahl M, Seada LS, Jänig U, Harms D, Klöppel G. Solid-pseudopapillary tumor of the pancreas: its origin revisited. Virchows Arch. 2000;436:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 226] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Lam KY, Lo CY, Fan ST. Pancreatic solid-cystic-papillary tumor: clinicopathologic features in eight patients from Hong Kong and review of the literature. World J Surg. 1999;23:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 170] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Santini D, Poli F, Lega S. Solid-papillary tumors of the pancreas: histopathology. JOP. 2006;7:131-136. [PubMed] |
| 13. | Wenig B, Heffess C, Adair C. In: Atlas of Endocrine Pathology. Philadelphia: Saunders/Elsevier 1997; 224-228. |
| 14. | Todani T, Shimada K, Watanabe Y, Toki A, Fujii T, Urushihara N. Frantz's tumor: a papillary and cystic tumor of the pancreas in girls. J Pediatr Surg. 1988;23:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Adsay NV, Klimstra DS. Cystic forms of typically solid pancreatic tumors. Semin Diagn Pathol. 2000;17:81-88. [PubMed] |
| 16. | Arai T, Kino I, Nakamura S, Koda K. Solid and cystic acinar cell tumors of the pancreas. A report of two cases with immunohistochemical and ultrastructural studies. Acta Pathol Jpn. 1986;36:1887-1896. [PubMed] |
| 17. | Murao T, Toda K, Tomiyama Y. Papillary and solid neoplasm of pancreas in a child. Report of a case in which acinar differentiation was demonstrated by immunohistochemistry and electron microscopy. Acta Pathol Jpn. 1983;33:565-575. [PubMed] |
| 18. | Lai HW, Su CH, Li AF, Wu LH, Shyr YM, Chen TH, Wu CW, Lui WY. Malignant solid and pseudopapillary tumor of the pancreas--clinicohistological, immunohistochemical, and flow cytometric evaluation. Hepatogastroenterology. 2006;53:291-295. [PubMed] |
| 19. | Mao C, Guvendi M, Domenico DR, Kim K, Thomford NR, Howard JM. Papillary cystic and solid tumors of the pancreas: a pancreatic embryonic tumor Studies of three cases and cumulative review of the world's literature. Surgery. 1995;118:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 189] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Ohtomo K, Furui S, Onoue M, Okada Y, Kusano S, Shiga J, Suda K. Solid and papillary epithelial neoplasm of the pancreas: MR imaging and pathologic correlation. Radiology. 1992;184:567-570. [PubMed] |
| 21. | Buetow PC, Buck JL, Pantongrag-Brown L, Beck KG, Ros PR, Adair CF. Solid and papillary epithelial neoplasm of the pancreas: imaging-pathologic correlation on 56 cases. Radiology. 1996;199:707-711. [PubMed] |
| 22. | Mergo PJ, Helmberger TK, Buetow PC, Helmberger RC, Ros PR. Pancreatic neoplasms: MR imaging and pathologic correlation. Radiographics. 1997;17:281-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 75] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Minami M, Itai Y, Ohtomo K, Yoshida H, Yoshikawa K, Iio M. Cystic neoplasms of the pancreas: comparison of MR imaging with CT. Radiology. 1989;171:53-56. [PubMed] |
| 24. | Kelekis NL, Semelka RC, Molina PL, Doerr ME. ACTH-secreting islet cell tumor: appearances on dynamic gadolinium-enhanced MRI. Magn Reson Imaging. 1995;13:641-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |