Published online Aug 14, 2006. doi: 10.3748/wjg.v12.i30.4908
Revised: April 11, 2006
Accepted: April 21, 2006
Published online: August 14, 2006
An estimated 300 million people worldwide suffer from chronic hepatitis C with a prevalence of 0.8%-1.0% of the general population in Canada. An increasing pool of evidence exists supporting the use of pegylated- interferon (pegIFN) and ribavirin combination therapy for hepatitis C. We report a 49-year old male of North American aboriginal descent with chronic hepatitis C (genotype 2b). Biopsy confirmed that he had cirrhosis with a 2-wk history of left eye pain and decreased visual acuity. He developed retinal vein thrombosis after 16 of 24 wk of pegIFN-α 2a and ribavirin combination therapy. He was urgently referred to a retinal specialist and diagnosed with non-ischemic central retinal vein occlusion of the left eye. PegIFN and ribavirin combination therapy was discontinued and HCV RNA was undetectable after 16 wk of treatment. Hematologic investigations revealed that the patient was a factor V Leiden heterozygote with mildly decreased protein C activity. Our patient had a number of hypercoagulable risk factors, including factor V Leiden heterozygosity, cirrhosis, and hepatitis C that alone would have most likely remained clinically silent. We speculate that in the setting of pegIFN treatment, these risk factors may coalesce and cause the retinal vein thrombosis.
- Citation: Zandieh I, Adenwalla M, Cheong-Lee C, Ma PE, Yoshida EM. Retinal vein thrombosis associated with pegylated-interferon and ribavirin combination therapy for chronic hepatitis C. World J Gastroenterol 2006; 12(30): 4908-4910
- URL: https://www.wjgnet.com/1007-9327/full/v12/i30/4908.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i30.4908
An estimated 300 million people worldwide suffer from chronic hepatitis C with a prevalence of 0.8%-1.0% of the general population in Canada[1]. In the last 10 years, dramatic advances have been made in the treatment of this common chronic condition. The pegylated-interferon (pegIFN) and ribavirin combination therapy has been shown to result in sustained virologic response rates of 46%-77%, depending on viral genotype[2]. Evidence has also emerged regarding the utility of interferon in cirrhotic hepatitis C treatment with reduced rates of both hepatocellular carcinoma and improved survival[3-5]. With the growing enthusiasm amongst patients and physicians alike, in favour of treatment as a result of the increasing pool of evidence supporting the use of interferon-based regimens, its adverse effects need to always be recognized and periodically reviewed.
Although interferon or pegIFN therapy can affect any organ system, the most commonly reported side effects include flu-like symptoms such as fever, chills, myalgia, fatigue, diarrhea, nausea and vomiting. Central nervous system disturbances including depression, suicidal ideation, confusion and mental status changes can occur, especially in patients with pre-existing histories. Hematologic side effects, including anemia, thrombocytopenia, and neutropenia, require ongoing monitoring. The reported withdrawal rates due to adverse effects, in studies examining interferon-based combinations are 7%-8%[2,6].
We report a case of central retinal vein thrombosis in a cirrhotic hepatitis C patient during pegIFN and ribavirin combination treatment.
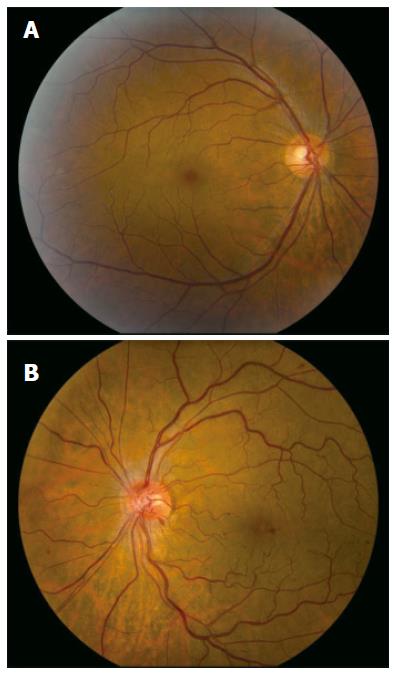
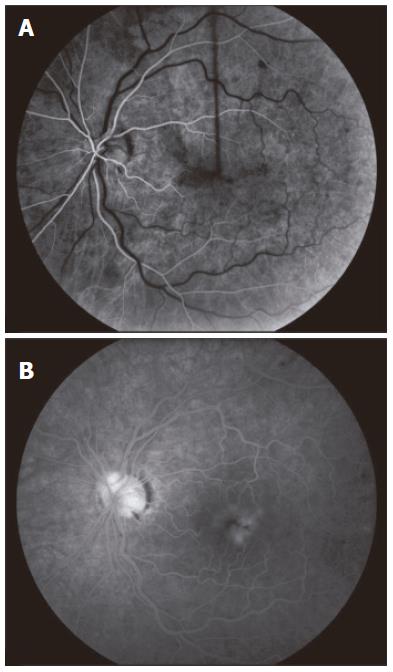
A 49-year old male of North American aboriginal descent, with chronic hepatitis C (genotype 2b) and biopsy confirmed cirrhosis, presented with a 2-wk history of left eye pain and decreased visual acuity, after a 16-24 wk course of therapy with pegIFN-α 2a at a dose of 180 μg per week injected subcutaneously and 800 mg ribavirin per day (Pegasys, Hoffmann-La Roche, Mississauga, ON, Canada). His past medical and family histories were negative for any thrombophilia. Specifically, he had no history of superficial or deep venous thrombosis and no history of thromboembolic events. Prior to the initiation of treatment, he had no evidence of decompensated liver disease and his serum alanine aminotransferase (ALT) was 256 U/L (upper limit of normal < 50U/L). Abdominal sonographic imaging revealed a cirrhotic liver with mild splenomegaly. Serial blood work performed at 4 and 8 wk of treatment revealed that his serum ALT levels were 67 U/L and 45 U/L (normal < 55 U/L), respectively. There were no complications associated with the treatment regimen prior to his presentation at 16 wk. One week following the onset of left eye pain and decreased visual acuity, he was assessed by an optometrist who prescribed eyeglasses. Due to the continued symptoms he presented to our hepatitis clinic, two weeks after the initial onset of symptoms. He was urgently referred to a retinal specialist and diagnosed with non-ischemic central retinal vein occlusion of the left eye (Figure 1). Fluorescein angiogram revealed delayed venous filling (Figure 2A) and associated macular edema (Figure 2B). Visual acuity at presentation was 20/20 in the right eye, and 20/70 in the left eye. PegIFN & ribavirin combination therapy was discontinued and the HCV RNA after 16 wk of treatment was undetectable.
Subsequent hematologic investigations to look for a hypercoagulable condition revealed heterozygosity for factor V Leiden, and a mildly decreased protein C activity at 0.49 U (lower limit of normal 0.65 U), confirmed by repeat testing. Antithrombin III and protein S levels were within normal limits but at the lower range with values of 0.72 U and 0.66 U, respectively (lower limits of normal for antithrombin III and protein S, 0.70 and 0.65 respectively). Cryoglobulins and the lupus anticoagulant were negative. Six months following the onset of our patient’s left eye pain, his visual deficit remained unchanged.
Interferons comprise a group of pleiotropic proteins with anti-viral, anti-inflammatory, and anti-angiogenesis characteristics. Interferons are also multifunctional immunoregulatory cytokines with effects at various points in the cytokine cascade, likely accounting for their immunostimulatory effects[7]. Due to their various mechanisms of action, interferons are well recognized to cause a variety of side effects as previously mentioned. From an ophthalmologic standpoint, there have been few reported cases of retinal vein thrombosis in patients treated with interferon or pegIFN. We are aware of only three reports in the medical literature[8-10]. Of these, two of the reports describe this rare complication in cirrhotic patients being treated for hepatitis C[9,10].
Regarding the potential thrombogenic properties of interferon, Guyer et al[11] in a 1993 paper reporting retinopathy, have suggested that IFN therapy may cause immune complex deposition in the retinal vasculature and leukocyte infiltration, leading to retinal ischemia, congestion, and hemorrhage. Interferon therapy has also been reported to induce a number of thrombogenic autoantibodies, including cryoglobulins, anti-nuclear, anti-smooth muscle, anti-liver-kidney microsomal, anti-thyroglobulin, and anti-phospholipid antibodies, which are thought to play a role in the pathogenesis of a hypercoagulable state[10].
In our patient, the causative role of pegylated-interferon therapy in inducing a hypercoaguable state that results in the retinal vein occlusion is strong given the temporal occurrence. However other risk factors may also have contributed. Considering the key role of the liver in coagulation, cirrhosis results in various impairments via multiple mechanisms: quantitative and qualitative platelet defects, decreased production of coagulation and inhibitor factors, vitamin K deficiency, synthesis of abnormal clotting factors, decreased clearance of activated factors by the reticuloendothelial system, hyperfibrinolysis, and disseminated intravascular coagulation. The natural inhibitors of coagulation, antithrombin III, protein C and protein S, were at low to borderline levels of activity in our patient. Nevertheless, it is important to note that plasma activity of inhibitors between 50% and 70% alone, is not associated with increased thrombotic events in cirrhotics, possibly because of the proportional impairment of procoagulants[12].
As part of the post diagnostic thrombophilic workup, our patient was found to be a heterozygote for the factor V Leiden mutation. Normally, factor V circulates in plasma as an inactive cofactor, awaiting activation by thrombin. Its inactivation requires protein C-mediated cleavage at arginine 306 and arginine 679. Genotypically, a point mutation in the gene-encoding factor V results in a missense mutation. The gene product, called factor V Leiden, which is not susceptible to cleavage by activated protein C, is inactivated more slowly and therefore confers an increased risk of venous thrombosis. The prevalence of heterozygosity for factor V Leiden is 5%-6% and is the most common inherited thrombophilia[13]. The lifetime risk of thrombosis in heterozygotes compared to patients with no defect has been found to range 2.2-4.9[14,15].
The hepatitis C virus itself has been found to induce a variety of potentially thrombogenic antibodies such as cryoglobulins, anti-nuclear, anti-smooth muscle, anti-cardiolipin and anti-phospholipid antibodies[16]. Since the envelope proteins of cytomegalovirus and herpes viruses have been reported to function as a source of procoagulant phospholipid, one could speculate that the hepatitis C envelope could also have procoagulant activity[17].
Our patient had a number of hypercoagulable risk factors that alone, most likely would have remained clinically silent. We speculate that in the setting of pegIFN treatment, these risk factors may coalesce and result in the retinal vein thrombosis. It is interesting that the three cases of retinal vein thrombosis described by Nadir et al[10] were being actively treated for hepatitis C with pegIFN, but were also found to have primary defects contributing to a hypercoagulable state including protein S deficiency, hyperhomocysteinemia, heterozygosity for factor V Leiden, anti-phospholipid and anti-cardiolipin antibodies. Therefore we conclude that pegIFN treatment is an important risk factor for the development of retinal vein thrombosis, however based on our case and those described in the literature, other underlying risk factors may also need to be present. We emphasize that retinal vein thrombosis is still a rare complication, and we would not advocate the routine thrombophilic work-up of patients being considered for pegIFN treatment. However, the diagnosis needs to be considered in any patient on pegIFN presenting with decreased visual acuity or eye pain and any patient on pegIFN therapy presenting with manifestiations of a thrombotic episode needs to undergo further hematologic investigation.
S- Editor Wang J L- Editor Wang XL E- Editor Bai SH
| 1. | Remis R, Hogg R, Krahn MD, Preiksaitis JK, Sherman M. Estimating the number of blood transfusion recipients infected by hepatitis C virus in Canada: 1960-1985 and 1990-1992. Report to Health Canada; . |
| 2. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4847] [Cited by in RCA: 4747] [Article Influence: 206.4] [Reference Citation Analysis (0)] |
| 3. | Shiratori Y, Ito Y, Yokosuka O, Imazeki F, Nakata R, Tanaka N, Arakawa Y, Hashimoto E, Hirota K, Yoshida H. Antiviral therapy for cirrhotic hepatitis C: association with reduced hepatocellular carcinoma development and improved survival. Ann Intern Med. 2005;142:105-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 207] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 4. | Mazzella G, Accogli E, Sottili S, Festi D, Orsini M, Salzetta A, Novelli V, Cipolla A, Fabbri C, Pezzoli A. Alpha interferon treatment may prevent hepatocellular carcinoma in HCV-related liver cirrhosis. J Hepatol. 1996;24:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 209] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Nishiguchi S, Kuroki T, Nakatani S, Morimoto H, Takeda T, Nakajima S, Shiomi S, Seki S, Kobayashi K, Otani S. Randomised trial of effects of interferon-alpha on incidence of hepatocellular carcinoma in chronic active hepatitis C with cirrhosis. Lancet. 1995;346:1051-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 607] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 6. | Reichard O, Norkrans G, Frydén A, Braconier JH, Sönnerborg A, Weiland O. Randomised, double-blind, placebo-controlled trial of interferon alpha-2b with and without ribavirin for chronic hepatitis C. The Swedish Study Group. Lancet. 1998;351:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 443] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 7. | Tilg H. New insights into the mechanisms of interferon alfa: an immunoregulatory and anti-inflammatory cytokine. Gastroenterology. 1997;112:1017-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 143] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Akyuz F, Akyuz U, Kocaman O, Kaymakoglu S. Rare complication of interferon alpha therapy: retinal vein thrombosis. Acta Gastroenterol Belg. 2005;68:394-395. [PubMed] |
| 9. | Rubio JE, Charles S. Interferon-associated combined branch retinal artery and central retinal vein obstruction. Retina. 2003;23:546-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Nadir A, Amin A, Chalisa N, van Thiel DH. Retinal vein thrombosis associated with chronic hepatitis C: a case series and review of the literature. J Viral Hepat. 2000;7:466-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Guyer DR, Tiedeman J, Yannuzzi LA, Slakter JS, Parke D, Kelley J, Tang RA, Marmor M, Abrams G, Miller JW. Interferon-associated retinopathy. Arch Ophthalmol. 1993;111:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 172] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Semin Liver Dis. 2002;22:83-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 245] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 13. | Ridker PM, Miletich JP, Hennekens CH, Buring JE. Ethnic distribution of factor V Leiden in 4047 men and women. Implications for venous thromboembolism screening. JAMA. 1997;277:1305-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 319] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 14. | Martinelli I, Mannucci PM, De Stefano V, Taioli E, Rossi V, Crosti F, Paciaroni K, Leone G, Faioni EM. Different risks of thrombosis in four coagulation defects associated with inherited thrombophilia: a study of 150 families. Blood. 1998;92:2353-2358. [PubMed] |
| 15. | Emmerich J, Rosendaal FR, Cattaneo M, Margaglione M, De Stefano V, Cumming T, Arruda V, Hillarp A, Reny JL. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism--pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Study Group for Pooled-Analysis in Venous Thromboembolism. Thromb Haemost. 2001;86:809-816. [PubMed] |
| 16. | Pawlotsky JM, Ben Yahia M, Andre C, Voisin MC, Intrator L, Roudot-Thoraval F, Deforges L, Duvoux C, Zafrani ES, Duval J. Immunological disorders in C virus chronic active hepatitis: a prospective case-control study. Hepatology. 1994;19:841-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 292] [Article Influence: 9.4] [Reference Citation Analysis (0)] |