Published online Aug 14, 2006. doi: 10.3748/wjg.v12.i30.4773
Revised: February 25, 2006
Accepted: March 10, 2006
Published online: August 14, 2006
Nuclear magnetic resonance spectroscopy allows the study of cellular biochemistry and metabolism, both in the whole body in vivo and at higher magnetic field strengths in vitro. Since the technique is non-invasive and non-selective, magnetic resonance spectroscopy methodologies have been widely applied in biochemistry and medicine. In vitro magnetic resonance spectroscopy studies of cells, body fluids and tissues have been used in medical biochemistry to investigate pathophysiological processes and more recently, the technique has been used by physicians to determine disease abnormalities in vivo. This highlighted topic illustrates the potential of in vitro magnetic resonance spectroscopy in studying the hepatobiliary system. The role of in vitro proton and phosphorus magnetic resonance spectroscopy in the study of malignant and non-malignant liver disease and bile composition studies are discussed, particularly with reference to correlative in vivo whole-body magnetic resonance spectroscopy applications. In summary, magnetic resonance spectroscopy techniques can provide non-invasive biochemical information on disease severity and pointers to underlying pathophysiological processes. Magnetic resonance spectroscopy holds potential promise as a screening tool for disease biomarkers, as well as assessing therapeutic response.
- Citation: Cox IJ, Sharif A, Cobbold JF, Thomas HC, Taylor-Robinson SD. Current and future applications of in vitro magnetic resonance spectroscopy in hepatobiliary disease. World J Gastroenterol 2006; 12(30): 4773-4783
- URL: https://www.wjgnet.com/1007-9327/full/v12/i30/4773.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i30.4773
The nuclear magnetic resonance (NMR) phenomenon was first reported in 1946[1] and has since been widely used by the scientific and medical communities. The 2003 Nobel Prize for Medicine was jointly awarded to Professor Sir Peter Mansfield and Professor Paul Lauterbur for their work on clinical NMR, and this serves to underline the impact on the medical community of the utility of this technique with all its diverse applications (http://www.nobel.se/medicine/laureates). In this highlight article, we explain the background to NMR and discuss various clinical studies that are relevant to the liver and the biliary system, with particular emphasis on in vitro applications that have analyzed body fluids, such as bile, or tissue obtained from liver biopsies.
NMR is a non-invasive and non-selective technique, allowing the study of molecular composition and structure. NMR forms the basis of magnetic resonance imaging (MRI) methodologies, which have been developed using whole body magnets. Spatial localization using magnetic field gradients has enabled very detailed images of the human body to be obtained. In vivo NMR spectroscopy studies can be performed as an adjunct to clinical MRI, as part of the same examination. Longitudinal studies can be readily undertaken using both of these MR applications. The term magnetic resonance spectroscopy (MRS) is used in this highlight topic to denote in vivo and ex vivo clinical NMR spectroscopy studies, and also encompasses in vitro NMR spectroscopy, performed in the laboratory at very high magnet field strengths.
Although a detailed knowledge of the physical principles that govern NMR is not essential for appreciating the scope of NMR applications, it is often helpful to have some understanding of some of the basic concepts that underlie these methodologies. The NMR technique exploits the behavior of atomic nuclei in an externally applied magnetic field. Magnetic resonance occurs because of the quantum mechanical property of “spin”, which is intrinsic to certain atomic nuclei. Examples of such nuclei of clinical relevance include hydrogen-1 (1H), carbon-13 (13C), fluorine-19 (19F), phosphorus-31 (31P) and chlorine-35 (35Cl). Such nuclei can be imagined to act like small bar magnets in a magnetic field by ‘lining up’ with or against the field, and can be excited by irradiation with non-ionizing radiofrequency (rf) energy. During relaxation following excitation, rf signals are generated which contain information regarding the magnetic environment experienced by each nucleus, and therefore about the molecules in which they exist. The resulting signals, the “free induction decay”, are detected by a receiver coil and can be expressed as a frequency spectrum by the mathematical process of Fourier transformation. The relative frequency position of a metabolite signal, its chemical shift, is dependent on the locally experienced magnetic field and therefore the local chemical environment. Consequently, each nucleus type within a molecule has a characteristic chemical shift. Hydrogen-1 (1H) and phosphorus-31 (31P) are the two nuclei most commonly used for biological studies, as they are ubiquitous in nature (1H = 99.985%, 31P = 100% natural abundance).
Jacobson and colleagues[2] used MRS to examine the effects of hydration of DNA in 1954. Current investigations include both in vitro MRS studies on tissue samples or body fluids and in vivo whole-body MRS studies.
In vitro MRS studies include the analysis of body fluids (such as plasma, urine or bile), extracts of tissue, or small biopsy-sized specimens of intact tissue. In vivo MRS is more difficult to perform and is characterized by poorer resolution of metabolites than in vitro MRS. This is due to factors such as the lower magnetic field strengths used for in vivo MRS clinical studies and also to the effects of magnetic susceptibility and patient motion. The typical magnetic field strength is 1.5-3.0 Tesla (T) for clinical MRS studies and 11.7-18.8 T for in vitro MRS studies.
In vitro MRS can detect and characterize a range of metabolic components simultaneously, even if their chemical identities are unknown at the time of analysis. In vitro MRS studies therefore provide a comprehensive metabolic profile of the low molecular weight components in biofluids and tissues, reflecting levels of endogenous metabolites involved in key cellular pathways, which indicate the physiological and pathophysiological status. The technique can also provide a profile of exogenous agents, including xenobiotics and their metabolites, and give an indication as to their effects on endogenous compounds[3]. For such reasons, in vitro MRS has become one of the most successful and popular techniques for biofluid analysis over the past 10 years[4].
It is relevant to consider in vitro MRS applications to the liver in the context of metabolite findings from both in vivo31P and 1H MRS studies of patients with liver disease, who have been examined using whole body MRI/MRS techniques. In vivo hepatic MRS studies have been primarily used in the research environment and have generally utilized 31P MRS[5], because 1H MRS is technically more challenging[6,7]. In vivo31P MRS gives information on cell turnover and energy state and has been used to grade patients with chronic liver disease[8] and to aid in diagnosis and treatment response in patients with cancer[9]. On the other hand, in vivo1H MRS allows quantification of intra-hepatocellular lipid (IHCL) levels, and therefore has been utilized recently to quantify the extent of steatosis in patients with fatty liver[10,11].
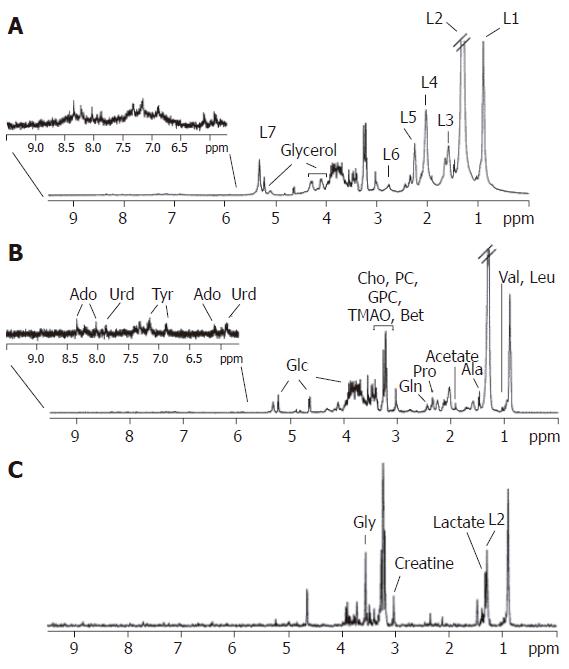
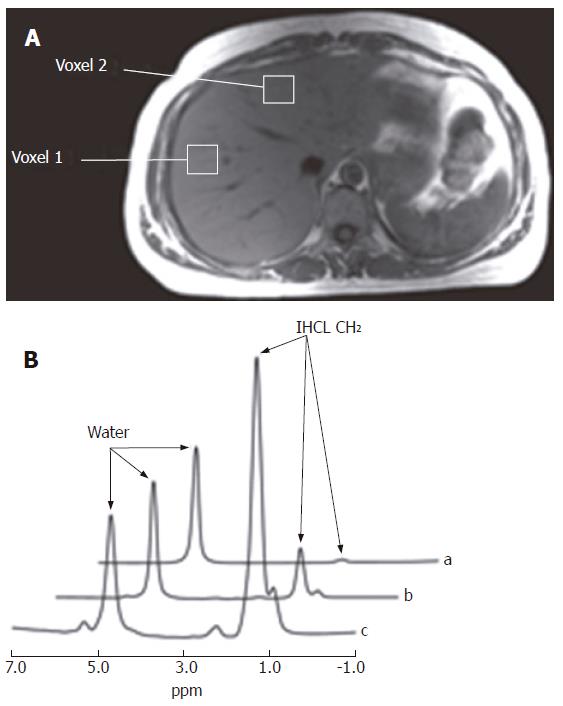
Representative hepatic 1H and 31P MR spectra are illustrated in Figures 1-5. In vitro1H and 31P MR spectra of liver tissue are illustrated in Figures 1 and 3, and these can be compared and contrasted with in vivo hepatic 1H and 31P MR spectra illustrated in Figures 2 and 4. An in vitro1H MR spectrum is dominated by contributions from water and IHCL. If the signal from water is suppressed using specific NMR methodologies, then more detailed contributions from IHCL can be observed along with resonances from choline-containing compounds (Cho) (Figures 1A-C). These data compare with the in vivo hepatic 1H MRS spectrum, which allows quantification of water and IHCL (Figure 2B).
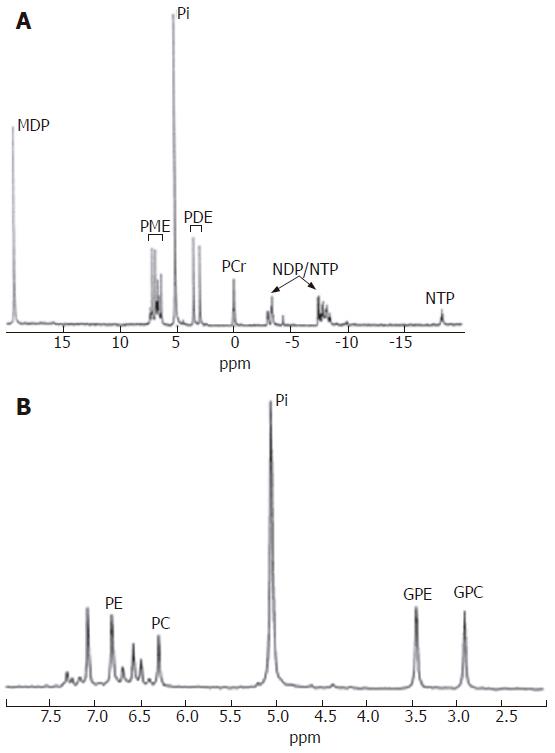
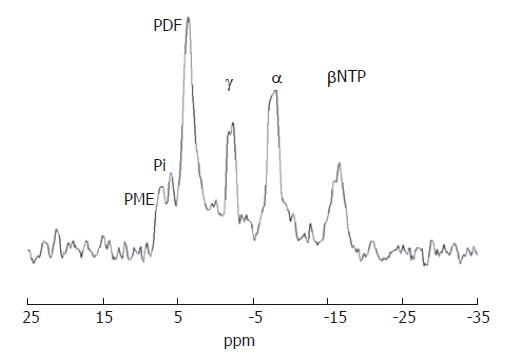
In vitro31P MR spectroscopy of liver tissue (Figure 3) allows characterization of resonances from phospholipid cell membrane precursors, including phosphocholine (PC) and phosphoethanolamnine (PE), adenosine monophosphate (AMP) and glycolytic intermediates, such as glucose-6-phosphate. Cell membrane degradation products, including glycerophosphorylcholine (GPC) and glycerophosphorylethanolamine (GPE) and mobile phospholipids from the endoplasmic reticulum can also be identified. Depending on how the tissue is collected it is also possible to quantify contributions to the spectrum from inorganic phosphate and various nucleoside triphosphates (NTP). Such spectral resolution is difficult to achieve in vivo, and therefore a typical in vivo hepatic 31P MR spectrum (Figure 4) consists of six dominant, composite resonances, arising from: (1) the phosphomonoester region (PME), which are mainly cell membrane precursors and sugar phosphates; (2) inorganic phosphate (Pi); (3) phosphodiesters (PDE), which are mainly cell membrane degradation products, but also signal from mobile phospholipids contained in mitochondria and (4-6) three resonances from NTP, which are chiefly composed of adenosine triphosphate (ATP), but contain contributions from uridine triphosphate, guanosine triphosphate and cytosine triphosphate[12].
Changes in the PME and PDE metabolites indicate modifications in rates of cell membrane synthesis, breakdown, cell death and regeneration, associated with increasingly rapid cell growth, turnover and development[13]. Thus, these important phosphorus-containing molecules are intricately involved in the cellular processes linked to cellular destruction, turnover and malignant transformation.
MRS also provides important insights into dynamic metabolic changes in the diseased liver. However, unlike most liver function tests, the information obtained is not dependent on blood flow. Furthermore, most standard liver function tests also depend on measurements of plasma components, rather than assessing markers at the site of disease. Recent studies on hepatitis C suggest that the MRS technique may be useful in monitoring response to treatment with interferon and ribavirin[8].
Sample preparation of body fluids requires routine clinical documentation to ensure that the relevant patient details are adequately controlled or accounted for, including time of sample collection and drug use history. It may be necessary to dilute or buffer the sample, or alternatively to document and account for any spectral changes relating to pH or concentration.
Sample storage should not cause a change in metabolite profile. Therefore storage temperature and conditions should be considered, and whether a change in sample temperature alters metabolite profile, for example during a freeze-thaw process[14,15].
Immediately prior to data collection, a known amount of a NMR standard can be added for NMR referencing and quantification. For example, sodium trimethylsilyl-[2H4] propionate (TSP) is routinely used as a reference compound for 1H MRS application. If the sample is required to be uncontaminated by any NMR reference compound for any further analysis, then either a reference compound can be placed in a capillary tube within the sample or an external standard is used.
The temperature of NMR data acquisition is under spectrometer control, so the sample temperature should be specified and constant throughout the study protocol. Many studies are performed at 25°C, but it may be necessary to study intact cells at lower temperatures to minimize the effects of ongoing cellular changes.
MR data acquisition includes adjusting the magnetic field homogeneity within the sample (a process known as ‘shimming’). Data averaging is generally required, so as to improve the signal-to-noise ratio in the spectrum. Data can be acquired using pulse-collection methods or one- or two-dimensional NMR data collection protocols[16]. The water signal is generally suppressed using saturation techniques and/or spin-echo methods, in order to more readily observe the metabolite signals[17]. Intact tissue specimens may be analyzed using conventional solution MR spectroscopy techniques, by simply placing the biopsy sample within a NMR tube or by chemically extracting aqueous or lipid soluble fractions of the tissues. Studying intact tissue specimens directly within a conventional NMR tube has the disadvantage that magnetic field inhomogeneities within the sample do limit the achievable spectral resolution, but has the advantage that sample preparation is minimal and the sample remains intact and available for further analysis by other complementary techniques. Tissue extract studies have the advantage that the sample which is finally available for MRS study that has taken place after the extraction process, has become homogeneous so that the spectral peaks are well defined. However, the obvious disadvantages include the total destruction of the sample, the additional influence of metabolite solubilities in the extract medium and the relatively large amount of tissue required (upwards of 100 mg). The recently introduced method of magic angle spinning (MAS) MRS can overcome the limitations of both of the above techniques[18].
The line-broadening effects in an intact semi-solid are caused by constraints on molecular motion from cellular architecture, chemical shift anisotropy and dipolar couplings, and these can be reduced by spinning the sample at an angle of 54.7º to the main magnetic field, known as the 'magic angle′. While such MAS MRS techniques have been used for many years in the NMR spectroscopy study of true solids, MAS MRS has only recently been applied to the study of intact tissue[18]. A distinct advantage is that a MAS MR spectrum can be obtained from as little as 2 mg tissue and the tissue remains sufficiently intact to allow subsequent histological or genetic analysis[19].
As in conventional MRS studies, accurate determination of metabolite concentrations is pivotal to the success of tissue MAS MRS studies. A number of factors need to be considered for metabolite quantitation in MAS MRS, including possible leakage of metabolites during washing of biopsies prior to analysis[15], temperature at which the spectrum is acquired and appropriate choice of reference standard. The question of how to define a standard for quantitation is particularly important. For example the tissue water signal measured from a proton spectrum without water presaturation, may be used as an internal standard. Silicone rubber, for example, provides a useful external standard. Alternatively peak area ratios can be used to compare different spectra, but this has limitations as with in vivo MRS studies. For example, not only can changes in absolute concentrations be missed by the use of metabolite ratios, but an increase in the area of one peak can have the same result on the area ratio as a decrease in the other. Therefore, relying on the ratio of signal areas may mask metabolite differences inherent in the spectra.
Owing to the ubiquity of phosphorus-containing moieties in energy metabolism, 31P MRS has been used to assess energy states in living systems[20]. However, the rapid degradation of phosphorylated nucleotide intermediates ex vivo, such as adenosine triphosphate (ATP) and adenosine diphosphate (ADP), has made snap-shot in vitro assessment difficult, although efforts have been made to minimize the ischemic time. Oxidation of these metabolites can occur at harvesting, snap freezing, thawing and during tissue extraction. In order to obtain metabolically useful information, the samples should be snap-frozen as soon as possible. In order to eliminate the need for the extraction of aqueous and lipid fractions, MAS MRS techniques could be used but data collection may need to be done at 4°C to minimise further metabolic reactions during data collection.
An alternative methodology to assess bioenergetics is to use an in vitro perfusion system. Applications have included the effects of normoxia and anoxia in rat livers[21], the response to cyanide intoxication[22] and the response of damaged liver to fructose loading[23].
31P and 1H MR spectra from aqueous soluble metabolites in extract of liver tumours of different types (hepatocellular carcinoma, colorectal metastases and secondary lung carcinoma) have been compared with samples from normal liver tissue and from histologically normal liver of the tumour host[24]. A significant increase in phosphoethanolamine (PER), phosphocholine (PC), taurine and lactate has been reported, with a reduction in GPE glycerophosphorylethanolamine (GPE) and glycerophosphorylcholine (GPC) in tumours, compared to normal liver controls[24]. In addition, the spectral changes identified by 31P MRS in tumour tissue have been seen at a lesser magnitude in the tumour host tissues[24].
Hepatic 31P MRS has been translated to the in vivo setting by Cox and colleagues and others[25]. The observed hepatic in vivo MR spectrum demonstrated a significantly raised PME/PDE ratio in tumour compared to controls. These peaks are thought to comprise increased PE and PC, and decreased GPE and GPC respectively, as elicited by in vitro analysis of tissue extract[24].
In vitro1H MRS in conjunction with a statistical classification strategy has been used by Soper and colleagues to differentiate histologically normal liver, cirrhotic nodules and hepatocellular carcinoma[26]. Multivariate and pattern recognition techniques enable all data points to be incorporated into the analysis, no matter whether the metabolites comprising the spectral peaks have been identified. In this study, cirrhotic nodules were distinguished from hepatocellular carcinoma in 98% of cases[26].
Initial hepatic MRS studies of chronic liver disease focused on cirrhosis of varying aetiologies. Human livers with histologically proven cirrhosis have been assessed using in vitro31P NMR spectroscopy[7]. Spectra from these patients with end-stage liver disease, all of whom had tissue obtained at the time of liver transplantation, showed significant elevations in phosphoethanolamine (PE) and phosphocholine (PC) and significant reductions in glycerophosphorylethanolamine (GPE) and glycerophosphorylcholine (GPC), when spectra were compared with those from histologically normal livers. Whether the patient had compensated or decompensated disease did not significantly alter the spectra obtained. Further work[27] by the same authors correlated such in vitro findings with 31P MRS in vivo and these studies suggest a potential clinical utility for in vivo MRS as an addendum to a standard MRI protocol in staging of the end-stage liver disease in patients that are being imaged for surveillance of hepatocellular carcinomas.
More recently, in vivo hepatic 31P MRS has been used to stratify inflammation and fibrosis caused by hepatitis C virus, compared to histological staging from standard liver biopsy[8]. The PME/PDE ratio was found to be elevated in mild, moderate/severe fibrosis and cirrhosis compared to normal, healthy volunteers. These changes have been thought to represent increased cell membrane turnover, although differences between means for each group were statistically significant, there was some overlap between the patient groups[8]. While histology remains the gold-standard, sampling error is inherent in the technique and it has been postulated that hepatic MRS could potentially provide a more accurate representation of the disease process, owing to the fact that it provides metabolite information from most of the liver[8]. Further studies directly correlating in vivo and in vitro MRS findings are awaited in this context.
While in vivo hepatic 31P MRS has been shown to correlate closely with disease severity in hepatitis C, its availability as a technique is not widespread. In vivo hepatic 1H MR spectroscopy would be more generally applicable on standard MRI scanners. Cho and colleagues have used this technique to stratify chronic hepatitis compared to histology[28]. They were able to detect differences between groups, but the signal-to-noise ratio was low and assignment of the spectral peaks is open to debate[29]. A rational approach would be to identify the change in spectral peaks in vitro, using 1H MAS MRS, prior to translating the technique to the in vivo setting. With this in mind, Martínez-Granados and colleagues performed 1H MAS MRS on needle biopsies of liver tissue from 16 patients with chronic hepatitis or cirrhosis and one specimen from autopsy, nominated a “normal” control. High quality spectra were obtained and a number of metabolites assigned to previously unidentified peaks. Data collection was carried out at 4°C to minimize tissue degradation. Differences in signal intensities between disease states and “normal” liver were noted, particularly increases in mobile fatty acids and glycogen in the former. However, the “normal” liver was harvested 24 h post mortem, so assumptions of metabolic normality are questionable[30].
The intrahepatocellular lipid (IHCL) component of lipid extracts from steatotic liver specimens as assessed by 1H MR spectroscopy has been calibrated with in vivo MR spectroscopy measurements to estimate the lipid volume fraction in fatty liver disease[31]. There is an agreement with CT estimations but histomorphometry appears to underestimate the fat volume in samples. Szczepaniak and colleagues used localised in vivo1H MRS in 2349 patients to assess the hepatic triglyceride content and estimated the prevalence of hepatic steatosis in the study group as 33.6%[32]. Further MRI studies in vivo have demonstrated a close relation between hepatic steatosis and body adiposity, and a close correlation between MRI estimation of adiposity and histological assessment in two of these patients[11]. Although studies have shown a close association between in vivo estimates and biopsies, in vitro MRS assessment of lipid content in liver biopsies by MAS MRS would allow direct comparison with histology, reducing the effect of sampling error[33].
Liver transplantation is used as a definitive therapy for acute hepatic failure, severe chronic liver disease and some cases of hepatocellular carcinoma. Demand for donor livers is high and suboptimal specimens may be used. At present there is no reliable non-invasive method to assess the viability of livers between organ harvesting and implantation. A program of research to address these problems demonstrates translational techniques from animal models in vitro to the clinical environment.
In vitro and ex vivo MRS studies of animals have established a model, which follows human organ harvesting and storage protocols[34,35]. Rapid reductions in ATP levels, readily measured by ex vivo31P MRS have been seen in a pig model compared to rodent models and levels could be replenished by hypothermic reperfusion[34]. Now that MR probes have been designed to fit within organ retrieval boxes, clinical studies of organs within a transplant program are required[36].
In a preliminary study, Duarte and colleagues used in vitro1H MAS MRS to assess biopsies taken at three time-points from six livers, before removal from donors, during cold perfusion and following implantation into the recipient[33]. The biopsies with the highest concentration of peaks reflecting fatty acyl chain (triglyceride) resonances were also identified as those also estimated to have the highest fat content on histological analysis. Other metabolites were identified, including glycerophosphocholine (GPC), which were reported to decrease from pre- to post-transplant[33]. However, in further studies such spectral changes need to be correlated with a range of clinical endpoints, including the premorbid clinical history of the liver donor, the pre-transplantation clinical history and nutritional status of the recipient, the subsequent post-transplantation liver function tests, pre-and post-transplantation indices of nutrition in the recipient and ultimately, the final clinical outcome.
Moving towards using MRS as a non-invasive methods for assessing graft dysfunction following transplantation, Taylor-Robinson and colleagues used correlative in vitro MRS of liver biopsy material and in vivo whole-body clinical hepatic 31P MRS to examine chronic ductopenic rejection of human liver allografts and noted an increased PME/NTP metabolite ratio reflecting associated altered phospholipid metabolism[37]. The study also included electron microscopy of liver tissue and the alteration in the phospholipid component was judged to be related to a change in biliary phospholipid excretion in these cholestatic patients[37]. Such an increase in the in vivo PDE resonance, seen in the in vivo hepatic MR spectrum is not a specific finding in patients with chronic allograft rejection, because a similar, albeit less marked change has been found in patients with primary biliary cirrhosis and obstetric cholestasis[38,39].
Iron overload may be a result of iatrogenic causes such as multiple blood transfusions in beta-thalassaemia, or of metabolic causes such as hereditary haemochromatosis, and is commonly associated with liver dysfunction. At the current time, hepatic iron stores are still usually estimated using liver biopsy with the associated risks that this procedure carries, but are readily studied by MR, since paramagnetic iron compounds cause magnetic inhomogeneities, which shorten the nuclear relaxation time[40,41]. However, imaging is difficult on account of rapid relaxation caused by these iron moieties. In addition, use of different MR sequences is required and equipment may require recalibration. Relaxometry allows measurement of relaxation times and provides information as to iron-proton interactions. An in vitro approach allows direct quantification of iron following MR analysis[42]. In vivo, Wang and colleagues demonstrated single voxel MRS measurement of T2 in liver iron overload that correlates strongly with iron quantification from biopsy and overcomes the difficulty of lack of detectable signals in conventional MRI[43]. Furthermore, Gandon and colleagues have proposed the use of a liver to muscle intensity ratio, which is transferable between equipment and sequences[44].
Gene therapy offers the opportunity to replace defective genes in phenotypically abnormal tissue with recombinant genes. Integration into the host cell genome allows expression of the desired protein. However, methods of conveying the gene to the required location and monitoring delivery and expression are required. Viral vectors and non-viral means such as naked DNA, liposomes and molecular conjugates have all been used. Expression varies with time and between tissues. MR techniques using MR spectroscopy and/or MR imaging, offer non-invasive methods of monitoring expression[45,46]. As in drug monitoring techniques where a MR detectable moiety or metabolite is linked to the active drug, genes expressing markers may be combined with the therapeutic gene. Phosphoarginine produced by the enzyme arginine kinase in Drosophila, but not present in mammalian skeletal muscle, is expressed in mouse skeletal muscle and detected by 31P MRS following injection of an adenovirus vector[47]. The expression in neonatal mice could continue for up to eight months. This demonstrates elegantly the principle of ‘marker metabolite’ using xenogenetic material. This principle has been demonstrated in vitro using a hepatocyte cell line[48]. Hepatocytes do not express creatinine kinase so phosphocreatine has not been seen on the hepatic MR spectrum. Integration of the cytoplasmic creatine kinase into hepatocytes in vitro could lead to detection of phosphocreatine by 31P MRS, raising the possibility that combined with a hepatotropic gene delivery system, gene expression may be monitored in vivo through a MR-visible marker.
Bile is predominantly an aqueous solution containing numerous constituents. Bile acids (BA), phosphatidylcholine (PTC) and cholesterol are the predominant lipid components of bile. Other components include electrolytes, organic anions (bilirubin), plasma proteins, hepatocyte proteins, peptides and amino acids, nucleotides, heavy metals and vitamins, xenobiotics and toxins[49]. In health the concentration of these biliary constituents is tightly controlled.
Primary bile acids such as chenodeoxycholic acid (CDCA) and cholic acid (CA), and secondary bile acids such as lithocholic acid (LCA) and deoxycholic acid (DOCA) are conjugated with the amino acids taurine and glycine and secreted as sodium or potassium salts into the biliary canaliculi via the ABC biliary transporter proteins[50]. Phosphatidylcholine, the predominant biliary phospholipid, is synthesized in hepatocytes and transported into the biliary canaliculi by the flippase multidrug resistant protein 3 (MDR3)[51]. Its main function is to form mixed micelles with primary and secondary bile acids and cholesterol essential for the emulsification of fats. More recently it has been shown to be cytoprotective to the biliary epithelium[52]. Cholesterol is solubilized by the formation of vesicles with PTC or mixed micelles with bile salts and PTC.
Sampling of bile for diagnostic purposes has become a common clinical practice since the introduction of endoscopic retrograde cholangiopancreatography (ERCP). Bile research has advanced in the last decade mainly as a consequence of the alarming rise in incidence of biliary tract cancer (cholangiocarcinoma)[53-55].
Advanced cytological techniques such as digital image analysis and fluoresence in situ hybridization (FISH) of bile are being used to improve the diagnostic accuracy of cholangiocarcinoma in certain experimental units[56]. More recently proteomic analysis of cholangiocarcinoma bile has identified 87 unique proteins including several novel proteins whose functions are unknown and a large number of proteins not previously described in bile[57]. Advances in molecular research and imaging technologies have vastly improved the diagnostic sensitivity and specificity in this area but there is still a clear need to identify novel, highly sensitive and specific biomarkers for fluid-based detection of biliary tract cancer, as well as other diseases of the biliary tree. Metabolic profiling of bile using in vitro MRS is a valuable experimental tool for the identification of such early biomarkers of the disease.
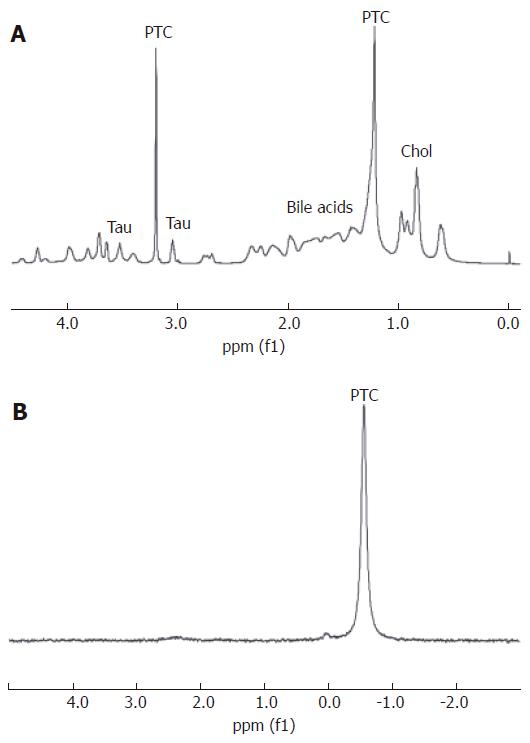
The majority of MRS studies on bile have been carried out using 1H MR spectroscopy. The hydrophobic association of conjugated bile acids and biliary lipids in micelles has been confirmed in early MR studies[58]. Bile acids have also been quantified in both animal and human bile using in vitro1H MRS. Rat bile studies with 1H MRS have derived peak assignments for C-18 methyl proton of bile acids at 0.7 ppm and the C-19 at 0.9 ppm, while the taurine moiety of taurine-conjugated bile acids resonated at 3.1 ppm and 3.5 ppm, respectively[59]. Conjugated bile acids in human gallbladder bile have been quantified using two dimensional MR[60]. Amide (-NH) proton resonances in glycine- and taurine-conjugated bile salts are in the region of 7.8-8.1 ppm[60].
More recently, 1H MRS has demonstrated cholesterol in human bile that can be differentiated from other lipid components in bile[61]. It has also been utilized for studying the effects of cholesterol on the fluidity of human gallbladder bile as well as for quantifying micellar PTC concentrations[61,62].
31P NMR spectroscopy has been used to quantify phospholipids in red cell membranes and in model bile salts[63]. Such 31P MRS studies have shown higher concentrations of PTC and Pi in gallbladder bile compared to canalicular bile[64].
The biliary epithelium is exposed to numerous constituents of bile. Bile acids, PTC and cholesterol are of particular importance in disease if the cytoprotecive mechanism of cholangiocytes is disrupted[65]. Biliary disease has a major effect on biliary composition as the level of cellular function determines the production and biochemical constituents of bile. Biliary composition of these lipids also varies in cholestatic diseases of the liver and malignancy of the biliary tree. Current applications of MRS in biliary disease include assessment of bile content in cholesterol gallstones, rejection in liver transplantation, primary biliary cirrhosis and biliary tract malignancy, as well in biliary excretion of xenobiotics[64,66-69].
MRS has been used to assess the distribution of biliary lipids between vesicles and micelles, which is believed to have a role in gallstone disease, and there is evidence that cholesterol from vesicular aggregates may be responsible for the deposition of cholesterol stones in the gallbladder[70]. Changes in the pattern of fatty acids of PTC with an increase of arachidonic acid have also been observed in bile from patients with gallstones[71]. Fusion and aggregation of phospholipid-cholesetrol vesicles which form liquid crystalline droplets leading to nucleation of cholesterol monohydrate crystals are thought to be responsible for the formation of cholesterol gallstones[70]. A selective reduction in biliary phospholipids has been suggested to be responsible for cholesterol gallstones in certain populations[72].
Initial 31P MRS studies on bile from patients with primary biliary cirrhosis have shown reduced levels of PTC and Pi when compared to bile from healthy volunteers[64], but the significance of these findings still remains to be determined in more extensive studies.
In vitro MRS has also been demonstrated to effectively identify and quantify xenobiotic metabolites in human and animal bile. Dioxins have been identified in biliary-cannulated rodents[67].
In vitro1H MRS analysis of bile has also been applied in human liver transplantation in an attempt to assess donor liver integrity. Melendez and colleagues studied twenty-four hepatic bile samples from eight liver donors[69]. The livers from two donors were steatotic on histological analysis, while the rest were normal. 1H MRS analysis could show more intense PTC resonances in bile from steatotic donor livers, compared to bile from histologically normal donor livers. Seventeen hepatic bile samples from four recipients collected immediately after donor liver reperfusion were also analyzed by 1H MRS and showed bile from donor livers with good early graft function had a progressive increase in the bile acid peaks which represents restoration of bile flow. When compared to grafts with early graft dysfunction, the relatively reduced bile acid peaks in these spectra suggested slow recovery of bile secretion. In this study a total quantification of bile acids was not feasible due overlapping signals from other biliary lipids, notably cholesterol and PTC[69].
Our preliminary studies have revealed differences in phospholipid metabolites that may help distinguish between malignant and non-malignant causes of pancreaticobiliary obstruction. A reduced PTC resonance was seen in the bile from the majority of patients with hepatobiliary cancer compared to bile from patients with non-malignant indications for ERCP. This preliminary observation was confirmed by significant differences in the peak area ratios of PTC, referenced to the TSP standard in the 1H MR spectra (P = 0.007)[68].
Nishijima and colleagues have observed a lactate peak in bile spectra from patients with hepatic and biliary malignancy but not in bile from patients with non-malignant disease or bile from healthy controls[73]. The significance of this finding has yet to be determined in larger studies, where the collection and storage of bile for analysis are performed according to uniform protocols without potential contamination from ERCP contrast agents or the uncertainty of a long storage time that may lead to lactate accumulation.
Although MRS is primarily a research tool and its use to study hepatobiliary disease is a relatively new area, the ability of both in vivo and in vitro MRS to provide quick, repetitive, and non-invasive assessments of organ function raises several possible future development areas, including non-invasive diagnosis and staging of disease.
In vitro MRS holds a particular promise in the metabolic profiling of body fluids, such as urine, plasma and bile to pinpoint the potential disease mechanisms and to assess the response of the body to treatment regimens. This is of particular relevance when patients are having in vivo MRS studies, since an in vivo/in vitro correlation of the metabolite profile obtained may highlight disease processes and inform further genetic profiling studies[39].
Several clinical areas may be of potential interest. In the oncology field, phospholipid profiling to assess changes by in vivo31P MRS may be useful in monitoring responses to the treatment of hepatic tumors, with co-temporaneous in vitro MRS analysis of plasma and urine. For example, chemoembolization of hepatic tumors has been found to correlate with a decrease in PME and increase in ATP concentrations using in vivo hepatic MRS[74].
Correlative in vitro MRS of body fluids and in vivo hepatic MRS techniques can also be applied to chronic liver disease to monitor liver fibrosis and progression towards cirrhosis. With increasing prevalence of alcohol-related liver disease, obesity-related liver disease (non-alcoholic hepatitis or NASH) and viral hepatitis, such as hepatitis C in particular, a reliable and repeatable monitoring system is required that obviates the morbidity and mortality associated with liver biopsy. In vivo and in vitro MRS may hold some of the answers to this, but further, larger scale studies are required to assess the true utility of these techniques with respect to other methodologies, such as microbubble contrast-enhanced ultrasound, ultrasound elastography and use of serological markers of fibrosis[75,76].
Future technical advances will boost the clinical potential of in vivo MRS, for example improving the delineation of multi-component signals, such as PME and PDE, with proton decoupling[77]. Increasing magnetic field strengths for in vivo studies may also provide better signal-to-noise and increased resolution, and 3T magnets are becoming steadily more available. Furthermore, in vivo1H MRS may find a role in monitoring hepatic lipid content in interventional treatment studies of patients with non-alcoholic fatty liver disease in the future. In vivo13C MRS for measurement of lipid metabolism, glycogen storage and gluconeogenesis looks promising for the future, but current sensitivity issues mean that this capability is currently confined only to a few centers[78-80]. In vitro MRS will find a correlative role in this context for screening plasma and urinary metabolites.
With respect to in vitro applications in bile, the ratio of taurine to glycine conjugates of bile acids and conjugated to unconjugated bile acids varies in hepatobiliary disease, and it has been widely accepted that elevated levels of bile acids in hepatocytes are toxic, leading to cholestasis[65]. More recently, the role of bile acids in cholangiocytes has been highlighted. In vitro studies on bile acids has demonstrated that they can act as ligands for the epidermal growth factor receptor on cholangiocytes and via the mitogen-activated protein kinase cell signalling pathway, leading to disordered cell cycling and cholangiocyte proliferation[81]. The measurement of bile acids is therefore necessary for an understanding of the pathophysiology of these diseases. In vitro MRS may find a useful role in screening bile in this context.
It is true that the significance of in vivo MRS needs to be assessed in larger studies with greater numbers of patients with various hepatobiliary diseases. Correlative in vitro NMR spectroscopy will help to elucidate some of the conundra. Trials in large populations in well-defined clinical settings are needed to determine if both in vivo and in vitro MRS can provide independent diagnostic and prognostic indices in management.
Some of the studies outlined were funded by the British Medical Research Council (MRC grant G99001978). Philips Medical Systems (Cleveland, Ohio, USA) are thanked for support of the magnet systems at the Robert Steiner MRI Unit, Hammersmith Hospital, Imperial College, London. We thank Adrian Lim, Shahid Khan and Nayna Patel for useful discussions.
S- Editor Wang J L- Editor Wang XL E- Editor Bi L
| 1. | Bloch F, Hansen WW, Packard ME. Nuclear induction. Physics Review. 1946;69:127. [RCA] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 742] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Jacobson B, Anderson WA, Arnold JT. A proton magnetic resonance study of the hydration of deoxyribonucleic acid. Nature. 1954;17:772-773. [RCA] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 79] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Lindon JC, Holmes E, Nicholson JK. Metabonomics and its role in drug development and disease diagnosis. Expert Rev Mol Diagn. 2004;4:189-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 120] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Bollard ME, Stanley EG, Lindon JC, Nicholson JK, Holmes E. NMR-based metabonomic approaches for evaluating physiological influences on biofluid composition. NMR Biomed. 2005;18:143-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 330] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 5. | Cox IJ, Menon DK, Sargentoni J, Bryant DJ, Collins AG, Coutts GA, Iles RA, Bell JD, Benjamin IS, Gilbey S. Phosphorus-31 magnetic resonance spectroscopy of the human liver using chemical shift imaging techniques. J Hepatol. 1992;14:265-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Cox IJ. Development and applications of in vivo clinical magnetic resonance spectroscopy. Prog Biophys Mol Biol. 1996;65:45-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Menon DK, Sargentoni J, Taylor-Robinson SD, Bell JD, Cox IJ, Bryant DJ, Coutts GA, Rolles K, Burroughs AK, Morgan MY. Effect of functional grade and etiology on in vivo hepatic phosphorus-31 magnetic resonance spectroscopy in cirrhosis: biochemical basis of spectral appearances. Hepatology. 1995;21:417-427. [PubMed] |
| 8. | Lim AK, Patel N, Hamilton G, Hajnal JV, Goldin RD, Taylor-Robinson SD. The relationship of in vivo 31P MR spectroscopy to histology in chronic hepatitis C. Hepatology. 2003;37:788-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Schilling A, Gewiese B, Berger G, Boese-Landgraf J, Fobbe F, Stiller D, Gallkowski U, Wolf KJ. Liver tumors: follow-up with P-31 MR spectroscopy after local chemotherapy and chemoembolization. Radiology. 1992;182:887-890. [PubMed] |
| 10. | Tanoli T, Yue P, Yablonskiy D, Schonfeld G. Fatty liver in familial hypobetalipoproteinemia: roles of the APOB defects, intra-abdominal adipose tissue, and insulin sensitivity. J Lipid Res. 2004;45:941-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Thomas EL, Hamilton G, Patel N, O’Dwyer R, Doré CJ, Goldin RD, Bell JD, Taylor-Robinson SD. Hepatic triglyceride content and its relation to body adiposity: a magnetic resonance imaging and proton magnetic resonance spectroscopy study. Gut. 2005;54:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 240] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Iles RA, Stevens AN, Griffiths JR, Morris PG. Phosphorylation status of liver by 31P-n.m.r. spectroscopy, and its implications for metabolic control. A comparison of 31P-n.m.r. spectroscopy (in vivo and in vitro) with chemical and enzymic determinations of ATP, ADP and Pi. Biochem J. 1985;229:141-151. [PubMed] |
| 13. | Ruiz-Cabello J, Cohen JS. Phospholipid metabolites as indicators of cancer cell function. NMR Biomed. 1992;5:226-233. [PubMed] [DOI] [Full Text] |
| 14. | Deprez S, Sweatman BC, Connor SC, Haselden JN, Waterfield CJ. Optimisation of collection, storage and preparation of rat plasma for 1H NMR spectroscopic analysis in toxicology studies to determine inherent variation in biochemical profiles. J Pharm Biomed Anal. 2002;30:1297-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Bourne R, Dzendrowskyj T, Mountford C. Leakage of metabolites from tissue biopsies can result in large errors in quantitation by MRS. NMR Biomed. 2003;16:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Tang H, Wang Y, Nicholson JK, Lindon JC. Use of relaxation-edited one-dimensional and two dimensional nuclear magnetic resonance spectroscopy to improve detection of small metabolites in blood plasma. Anal Biochem. 2004;325:260-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Chen JH, Sambol EB, Kennealey PT, O’Connor RB, DeCarolis PL, Cory DG, Singer S. Water suppression without signal loss in HR-MAS 1H NMR of cells and tissues. J Magn Reson. 2004;171:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Cheng LL, Lean CL, Bogdanova A, Wright SC, Ackerman JL, Brady TJ, Garrido L. Enhanced resolution of proton NMR spectra of malignant lymph nodes using magic-angle spinning. Magn Reson Med. 1996;36:653-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 143] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Dave U, Taylor-Robinson SD, Walker MM, Mahon M, Puri BK, Thursz MR, DeSouza NM, Cox IJ. In vitro 1H-magnetic resonance spectroscopy of Barrett’s esophageal mucosa using magic angle spinning techniques. Eur J Gastroenterol Hepatol. 2004;16:1199-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Hoult DI, Busby SJ, Gadian DG, Radda GK, Richards RE, Seeley PJ. Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature. 1974;252:285-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 378] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 21. | McLaughlin AC, Takeda H, Chance B. Rapid ATP assays in perfused mouse liver by 31P NMR. Proc Natl Acad Sci U S A. 1979;76:5445-5449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Salhany JM, Stohs SJ, Reinke LA, Pieper GM, Hassing JM. 31P nuclear magnetic resonance of metabolic changes associated with cyanide intoxication in the perfused rat liver. Biochem Biophys Res Commun. 1979;86:1077-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Thoma WJ, Uğurbil K. Effect of adenine on liver nucleotide after fructose loading by 31P-NMR. Am J Physiol. 1989;256:G949-G956. [PubMed] |
| 24. | Bell JD, Cox IJ, Sargentoni J, Peden CJ, Menon DK, Foster CS, Watanapa P, Iles RA, Urenjak J. A 31P and 1H-NMR investigation in vitro of normal and abnormal human liver. Biochim Biophys Acta. 1993;1225:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Cox IJ, Bell JD, Peden CJ, Iles RA, Foster CS, Watanapa P, Williamson RC. In vivo and in vitro 31P magnetic resonance spectroscopy of focal hepatic malignancies. NMR Biomed. 1992;5:114-120. [PubMed] [DOI] [Full Text] |
| 26. | Soper R, Himmelreich U, Painter D, Somorjai RL, Lean CL, Dolenko B, Mountford CE, Russell P. Pathology of hepatocellular carcinoma and its precursors using proton magnetic resonance spectroscopy and a statistical classification strategy. Pathology. 2002;34:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Taylor-Robinson SD, Sargentoni J, Bell JD, Saeed N, Changani KK, Davidson BR, Rolles K, Burroughs AK, Hodgson HJ, Foster CS. In vivo and in vitro hepatic 31P magnetic resonance spectroscopy and electron microscopy of the cirrhotic liver. Liver. 1997;17:198-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Cho SG, Kim MY, Kim HJ, Kim YS, Choi W, Shin SH, Hong KC, Kim YB, Lee JH, Suh CH. Chronic hepatitis: in vivo proton MR spectroscopic evaluation of the liver and correlation with histopathologic findings. Radiology. 2001;221:740-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Lim AK, Hamilton G, Patel N, Bell JD, Taylor-Robinson SD. H MR spectroscopy in the evaluation of the severity of chronic liver disease. Radiology. 2003;226:288-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Martínez-Granados B, Monleón D, Martínez-Bisbal MC, Rodrigo JM, del Olmo J, Lluch P, Ferrández A, Martí-Bonmatí L, Celda B. Metabolite identification in human liver needle biopsies by high-resolution magic angle spinning 1H NMR spectroscopy. NMR Biomed. 2006;19:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Longo R, Pollesello P, Ricci C, Masutti F, Kvam BJ, Bercich L, Crocè LS, Grigolato P, Paoletti S, de Bernard B. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J Magn Reson Imaging. 1995;5:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 298] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 32. | Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462-E468. [PubMed] |
| 33. | Duarte IF, Stanley EG, Holmes E, Lindon JC, Gil AM, Tang H, Ferdinand R, McKee CG, Nicholson JK, Vilca-Melendez H. Metabolic assessment of human liver transplants from biopsy samples at the donor and recipient stages using high-resolution magic angle spinning 1H NMR spectroscopy. Anal Chem. 2005;77:5570-5578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Changani KK, Fuller BJ, Bell JD, Bryant DJ, Moore DP, Taylor-Robinson SD, Davidson BR. Hepatic nucleotide triphosphate regeneration after hypothermic reperfusion in the pig model: an in vitro P-NMR study. Transplantation. 1996;62:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Changani KK, Fuller BJ, Bryant DJ, Bell JD, Ala-Korpela M, Taylor-Robinson SD, Moore DP, Davidson BR. Non-invasive assessment of ATP regeneration potential of the preserved donor liver. A 31P MRS study in pig liver. J Hepatol. 1997;26:336-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Davidson BR, Barnard ML, Changani KK, Taylor-Robinson SD. Liver transplantation: current and potential applications of magnetic resonance spectroscopy. Liver Transpl Surg. 1997;3:481-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Taylor-Robinson SD, Sargentoni J, Bell JD, Thomas EL, Marcus CD, Changani KK, Saeed N, Hodgson HJ, Davidson BR, Burroughs AK. In vivo and in vitro hepatic phosphorus-31 magnetic resonance spectroscopy and electron microscopy in chronic ductopenic rejection of human liver allografts. Gut. 1998;42:735-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Jalan R, Sargentoni J, Coutts GA, Bell JD, Rolles K, Burroughs AK, Taylor Robinson SD. Hepatic phosphorus-31 magnetic resonance spectroscopy in primary biliary cirrhosis and its relation to prognostic models. Gut. 1996;39:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 39. | Müllenbach R, Bennett A, Tetlow N, Patel N, Hamilton G, Cheng F, Chambers J, Howard R, Taylor-Robinson SD, Williamson C. ATP8B1 mutations in British cases with intrahepatic cholestasis of pregnancy. Gut. 2005;54:829-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Stark DD, Moseley ME, Bacon BR, Moss AA, Goldberg HI, Bass NM, James TL. Magnetic resonance imaging and spectroscopy of hepatic iron overload. Radiology. 1985;154:137-142. [PubMed] |
| 41. | Stark DD, Moss AA, Goldberg HI. Nuclear magnetic resonance of the liver, spleen, and pancreas. Cardiovasc Intervent Radiol. 1986;8:329-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Ghugre NR, Coates TD, Nelson MD, Wood JC. Mechanisms of tissue-iron relaxivity: nuclear magnetic resonance studies of human liver biopsy specimens. Magn Reson Med. 2005;54:1185-1193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Wang ZJ, Haselgrove JC, Martin MB, Hubbard AM, Li S, Loomes K, Moore JR, Zhao H, Cohen AR. Evaluation of iron overload by single voxel MRS measurement of liver T2. J Magn Reson Imaging. 2002;15:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Gandon Y, Olivié D, Guyader D, Aubé C, Oberti F, Sebille V, Deugnier Y. Non-invasive assessment of hepatic iron stores by MRI. Lancet. 2004;363:357-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 475] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 45. | Bell JD, Taylor-Robinson SD. Assessing gene expression in vivo: magnetic resonance imaging and spectroscopy. Gene Ther. 2000;7:1259-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Bhakoo KK, Bell JD, Cox IJ, Taylor-Robinson SD. The application of magnetic resonance imaging and spectroscopy to gene therapy. Methods Enzymol. 2004;386:303-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 47. | Walter G, Barton ER, Sweeney HL. Noninvasive measurement of gene expression in skeletal muscle. Proc Natl Acad Sci U S A. 2000;97:5151-5155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Li Z, Qiao H, Lebherz C, Choi SR, Zhou X, Gao G, Kung HF, Rader DJ, Wilson JM, Glickson JD. Creatine kinase, a magnetic resonance-detectable marker gene for quantification of liver-directed gene transfer. Hum Gene Ther. 2005;16:1429-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Schiff ER, Sorrell MF, Maddrey WC. Schiff‘s Diseases of the Liver, 9th ed. Philadelphia: Lippincott Williams & Wilkins 2002; 141. |
| 50. | Wolkoff AW, Cohen DE. Bile acid regulation of hepatic physiology: I. Hepatocyte transport of bile acids. Am J Physiol Gastrointest Liver Physiol. 2003;284:G175-G179. [PubMed] |
| 51. | van Helvoort A, Smith AJ, Sprong H, Fritzsche I, Schinkel AH, Borst P, van Meer G. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell. 1996;87:507-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 625] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 52. | Komichi D, Tazuma S, Nishioka T, Hyogo H, Une M, Chayama K. Unique inhibition of bile salt-induced apoptosis by lecithins and cytoprotective bile salts in immortalized mouse cholangiocytes. Dig Dis Sci. 2003;48:2315-2322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Taylor-Robinson SD, Toledano MB, Arora S, Keegan TJ, Hargreaves S, Beck A, Khan SA, Elliott P, Thomas HC. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968-1998. Gut. 2001;48:816-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 310] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 54. | Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 421] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 55. | Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 899] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 56. | Kipp BR, Stadheim LM, Halling SA, Pochron NL, Harmsen S, Nagorney DM, Sebo TJ, Therneau TM, Gores GJ, de Groen PC. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. Am J Gastroenterol. 2004;99:1675-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 254] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 57. | Kristiansen TZ, Bunkenborg J, Gronborg M, Molina H, Thuluvath PJ, Argani P, Goggins MG, Maitra A, Pandey A. A proteomic analysis of human bile. Mol Cell Proteomics. 2004;3:715-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 58. | Small DM, Penkett SA, Chapman D. Studies on simple and mixed bile salt micelles by nuclear magnetic resonance spectroscopy. Biochim Biophys Acta. 1969;176:178-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 292] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 59. | Ishikawa H, Nakashima T, Inaba K, Mitsuyoshi H, Nakajima Y, Sakamoto Y, Okanoue T, Kashima K, Seo Y. Proton magnetic resonance assay of total and taurine-conjugated bile acids in bile. J Lipid Res. 1999;40:1920-1924. [PubMed] |
| 60. | Ijare OB, Somashekar BS, Gowda GA, Sharma A, Kapoor VK, Khetrapal CL. Quantification of glycine and taurine conjugated bile acids in human bile using 1H NMR spectroscopy. Magn Reson Med. 2005;53:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 61. | Ellul JP, Murphy GM, Parkes HG, Slapa RZ, Dowling RH. Nuclear magnetic resonance spectroscopy to determine the micellar cholesterol in human bile. FEBS Lett. 1992;300:30-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Groen AK, Goldhoorn BG, Egbers PH, Chamuleau RA, Tytgat GN, Bovée WM. Use of 1H-NMR to determine the distribution of lecithin between the micellar and vesicular phases in model bile. J Lipid Res. 1990;31:1315-1321. [PubMed] |
| 63. | Pearce JM, Komoroski RA. Resolution of phospholipid molecular species by 31P NMR. Magn Reson Med. 1993;29:724-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 64. | Khristianovich DS, Reshetniak VI, Loginov AS, Iushmanov VE, Tumanian MA. [31P-NMR spectroscopy of the human liver and bile]. Biull Eksp Biol Med. 1988;106:678-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 65. | Danchenko E, Petermann H, Chirkin A, Dargel R. Effect of bile acids on the proliferative activity and apoptosis of rat hepatocytes. Exp Toxicol Pathol. 2001;53:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 66. | Sequeira SS, Parkes HG, Ellul JP, Murphy GM. One dimensional high resolution 1H NMR spectroscopy of human bile: lipid-derived resonance shifts. Biochem Soc Trans. 1994;22:114S. [PubMed] |
| 67. | Hakk H, Larsen G, Feil V. Tissue distribution, excretion, and metabolism of 1,2,7,8-tetrachlorodibenzo-p-dioxin in the rat. Chemosphere. 2001;42:975-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 68. | Khan SA, Cox IJ, Thillainayagam AV, Bansi DS, Thomas HC, Taylor-Robinson SD. Proton and phosphorus-31 nuclear magnetic resonance spectroscopy of human bile in hepatopancreaticobiliary cancer. Eur J Gastroenterol Hepatol. 2005;17:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 69. | Melendez HV, Ahmadi D, Parkes HG, Rela M, Murphy G, Heaton N. Proton nuclear magnetic resonance analysis of hepatic bile from donors and recipients in human liver transplantation. Transplantation. 2001;72:855-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 70. | Jüngst D, Lang T, Huber P, Lange V, Paumgartner G. Effect of phospholipids and bile acids on cholesterol nucleation time and vesicular/micellar cholesterol in gallbladder bile of patients with cholesterol stones. J Lipid Res. 1993;34:1457-1464. [PubMed] |
| 71. | Cantafora A, Angelico M, Di Biase A, Pièche U, Bracci F, Attili AF, Capocaccia L. Structure of biliary phosphatidylcholine in cholesterol gallstone patients. Lipids. 1981;16:589-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 72. | Nervi F, Covarrubias C, Bravo P, Velasco N, Ulloa N, Cruz F, Fava M, Severín C, Del Pozo R, Antezana C. Influence of legume intake on biliary lipids and cholesterol saturation in young Chilean men. Identification of a dietary risk factor for cholesterol gallstone formation in a highly prevalent area. Gastroenterology. 1989;96:825-830. [PubMed] |
| 73. | Nishijima T, Nishina M, Fujiwara K. Measurement of lactate levels in serum and bile using proton nuclear magnetic resonance in patients with hepatobiliary diseases: its utility in detection of malignancies. Jpn J Clin Oncol. 1997;27:13-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Meyerhoff DJ, Karczmar GS, Valone F, Venook A, Matson GB, Weiner MW. Hepatic cancers and their response to chemoembolization therapy. Quantitative image-guided 31P magnetic resonance spectroscopy. Invest Radiol. 1992;27:456-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 75. | Lim AK, Taylor-Robinson SD, Patel N, Eckersley RJ, Goldin RD, Hamilton G, Foster GR, Thomas HC, Cosgrove DO, Blomley MJ. Hepatic vein transit times using a microbubble agent can predict disease severity non-invasively in patients with hepatitis C. Gut. 2005;54:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 76. | Colletta C, Smirne C, Fabris C, Toniutto P, Rapetti R, Minisini R, Pirisi M. Value of two noninvasive methods to detect progression of fibrosis among HCV carriers with normal aminotransferases. Hepatology. 2005;42:838-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 158] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 77. | Zakian KL, Koutcher JA, Malhotra S, Thaler H, Jarnagin W, Schwartz L, Fong Y. Liver regeneration in humans is characterized by significant changes in cellular phosphorus metabolism: assessment using proton-decoupled 31P-magnetic resonance spectroscopic imaging. Magn Reson Med. 2005;54:264-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Dobbins RL, Malloy CR. Measuring in-vivo metabolism using nuclear magnetic resonance. Curr Opin Clin Nutr Metab Care. 2003;6:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 79. | Flück CE, Slotboom J, Nuoffer JM, Kreis R, Boesch C, Mullis PE. Normal hepatic glycogen storage after fasting and feeding in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2003;4:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 80. | Roden M, Petersen KF, Shulman GI. Nuclear magnetic resonance studies of hepatic glucose metabolism in humans. Recent Prog Horm Res. 2001;56:219-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 81. | Komichi D, Tazuma S, Nishioka T, Hyogo H, Chayama K. A nuclear receptor ligand down-regulates cytosolic phospholipase A2 expression to reduce bile acid-induced cyclooxygenase 2 activity in cholangiocytes: implications of anticarcinogenic action of farnesoid X receptor agonists. Dig Dis Sci. 2005;50:514-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |