Published online Jul 14, 2006. doi: 10.3748/wjg.v12.i26.4214
Revised: December 2, 2005
Accepted: December 7, 2005
Published online: July 14, 2006
AIM: To observe the dynamic changes of liver microcirculation in vivo after arterial embolization with degradable starch microspheres (DSM).
METHODS: DSM were injected into the proper hepatic artery through a silastic tube inserted retrogradely in gastroduodenal artery (GDA) of SD rats. Fluorescent microscopy was used to evaluate the dynamic changes of blood flow through the terminal portal venules (TPVs), sinusoids and terminal hepatic venules (THVs). The movements of DSM debris were also recorded. Six hours after injection of DSM, percentages of THVs with completely stagnant blood flow were recorded.
RESULTS: Two phases of blood flow change were recorded. In phase one: after intra-arterial injection of DSM, slow or stagnant blood flow was immediately recorded in TPVs, sinusoids and THVs. This change was reversible, and blood flow resumed completely. In phase two: after phase one, blood flow in TPVs changed again and three patterns of blood flow were recorded. Six hours after DSM injection, 36.9% ± 9.2% of THVs were found with completely stagnant blood flow.
CONCLUSION: DSM can stop the microcirculatory blood flow in some areas of liver parenchyma. Liver parenchyma supplied by arteries with larger A-P shunt is considered at a higher risk of total microcirculatory blood stagnation after injection of DSM through hepatic artery.
-
Citation: Wang J, Murata S, Kumazaki T. Liver microcirculation after hepatic artery embolization with degradable starch microspheres
in vivo . World J Gastroenterol 2006; 12(26): 4214-4218 - URL: https://www.wjgnet.com/1007-9327/full/v12/i26/4214.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i26.4214
It has been accepted that transarterial chemoembolization (TACE) is an effective method to treat the unresectable hepatocellular carcinoma (HCC). With the characteristics of long retention time in the tumor tissue, iodinated poppyseed oil (Lipiodol) has been frequently used as the embolization material in clinical practice[1-4]. Animal experiments demonstrated that after injection of iodized oil into the hepatic artery, small Lipiodol drops could be found in the terminal portal venules (TPVs), which was assumed passing through the pathway of arterial-portal anastomosis such as the peribiliary pluxes[5-7]. When Lipiodol drops flow into the sinusoids, they can severely occlude the blood flow, cause the stagnation of local microcirculation, and further lead to ischemic liver parenchyma injury[8,9]. Super-selective technique with microcatheter and guidewires has been considered as a safe and effective way to treat HCC. Under some conditions in which liver function is severely damaged or blood supply of HCC is so complex that it is impossible to super-select the tumor feeding arteries, TACE is developed. Degradable starch microspheres (DSM), a temporary artery embolizer has been increasingly used as an alternative to Lipiodol in some particular situations[10-12]. It has also been suggested that, when the tumor feeding artery cannot be super-selected by microcatheter and guidewire, one-shot injection of DSM before TACE can be regarded as a practical method to protect the tumor free liver tissue from the injury caused by Lipiodol inflow following TAE. To fully understand its effects on liver microcirculation, we injected DSM through proper hepatic artery of rats, and the dynamic changes of liver microcirculation were evaluated by in vivo fluorescent microscopic observations.
Ten Sprague-Dawley rats weighing 300 to 450 g were used in compliance with the regulations and the Guide for the Care and Use of Laboratory Animals. The animals were fed with standard food pellets and tap water ad libitum. They were deprived of food but obtained free access to water for 12 h before the experiments. Anesthesia was performed by intra-peritoneal injection of 50 mg/kg sodium pentobarbital. The left femoral vein was cannulated with a 1F silastic tube (Natsume Corp. Tokyo, Japan) for additional anesthesia and liquid transfusion during the procedure. After a midline abdominal incision, the liver was carefully retracted to expose the gastroduodenal artery (GDA), which was catheterized by another 1F silastic tube (Natsume Corp., Tokyo, Japan) with its tip placed before the bifurcation that leads to the proper hepatic artery. The left lobe of liver was gently exteriorized and positioned over the window of the microscope stage. The liver parenchyma was covered with a small piece of plastic wrap; its surface was constantly irrigated with Ringer’s solution at the body temperature.
The exteriorized left liver lobe was transilluminated with monochromatic light generated by a prism monochromator equipped with a xenon lamp. Microscopic images of the microvasculatures were obtained with objective lenses (magnification, × 10, × 20) and an ocular lens (magnification, × 10). DSM (Yakult Honsha Co., Ltd., Tokyo, Japan) 12 mg in 0.2 mL was prepared in a 1 mL syringe and the solution was made uniform before injection. After infusion of 1 g/L fluorescent sodium 0.1 mL into the cannulated GDA, DSM was injected gently in one minute. The in vivo microscopic images of the following procedure were recorded on videotapes.
For each rat, areas with best visualization were selected for evaluation. Six hours later, 1 g/L fluorescent sodium 0.1 mL was infused through the cannulated left femoral vein to check the whole surface of liver lobe for a complete confirmation of the liver microcirculation. One hundred THVs were randomly selected during the horizontal and vertical movements of the microscope. THVs with completely stopped blood flow were statistically counted.
Data analysis was performed employing the Statistical Package for the Social Sciences Version 12.0 for Windows (SPSS© Inc., Chicago IL, USA). Results of the descriptive statistical analysis were presented as mean ± SD.
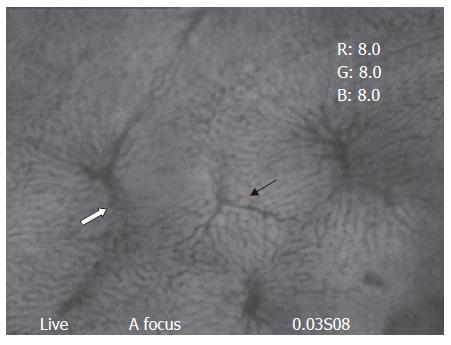
Clear images of the liver microcirculation (TPVs, sinusoids, and THVs) could be seen under in vivo fluorescent microscope (Figure 1). Blood flow from one TPV was drained through the sinusoids into several THVs; similarly, one particular THV provided venular drainage for several TPVs. Hepatic arterioles, the other afferent vessels in the liver, usually could not be visualized (Figure 2).
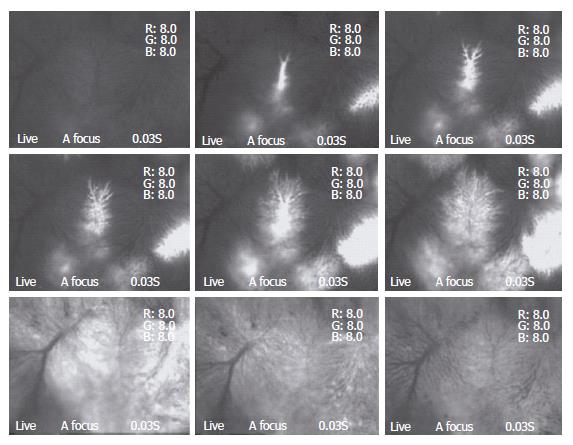
Blood flow through TPVs demonstrated an immediate response after DSM injection. The speed of blood flow dropped dramatically at once. In 2.0 ± 0.8 min (Max 3 min), the blood flow in the observed area completely stopped. After that, blood flow through TPVs resumed gradually; 12.7 ± 2.1 min (Max 15 min) after injection of the DSM, blood flow through TPVs completely recovered (Table 1). No evidence of DSM or its disaggregated debris could be recorded within this time interval. For convenient explanation, we named this period as “phase one”, and the blood flow changes afterward as “phase two”. In phase two, three different types of blood flow changes in TPVs could be recorded.
| No. | Start of bloodflow stagnation | Time of completerecovery of blood flow |
| 1 | 1.1 | 10.9 |
| 2 | 1.4 | 13.1 |
| 3 | 2.6 | 15 |
| 4 | 1 | 14.9 |
| 5 | 1.6 | 9.82 |
| 6 | 2.3 | 11.5 |
| 7 | 2.8 | 13.2 |
| 8 | 3 | 14.5 |
| 9 | 2.8 | 14.3 |
| 10 | 1.6 | 9.47 |
| mean ± SD | 2.0 ± 0.8 | 12.7 ± 2.1 |
Type one: The speed of blood flow slowed down again, and then completely stopped. This phenomenon could be found as early as 25 min after DSM injection and last for the whole procedure. The TPVs could never resume their blood flow during the observational period. DSM or its debris could be hardly found in TPVs in this type.
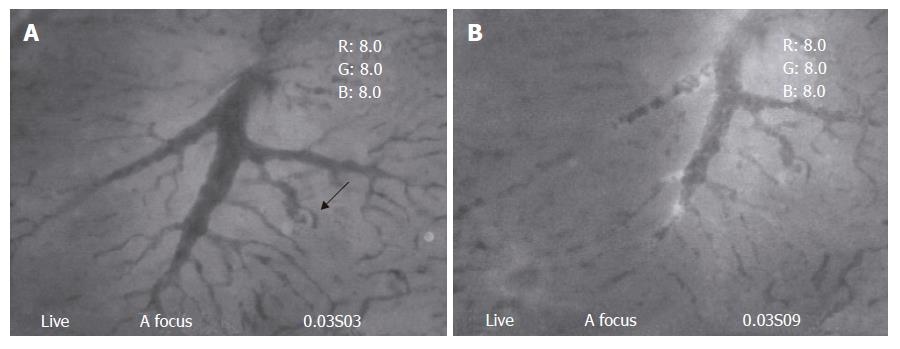
Type two: The speed of blood flow in TPVs decreased at different level, either slightly striking or some times intermittent, however the stagnation was not recorded during the whole observation time. Numerous small pieces of debris with irregular shapes could be found to flow through and drain into the distal sinusoids (Figure 3A and B).
Type three: In particular areas, TPVs kept a constant flow after phase one. The blood flow did not change during the whole procedure. DSM or its debris could not be recorded in these TPVs.
The blood flow through sinusoids and THVs followed the changes of TPVs in phase one. They also demonstrated a dramatic decrease of blood flow speed after DSM injection. Some even completely stopped. Nevertheless, it could be fully recovered.
During the following period, three kinds of blood flow, similar to that of the TPVs could also be recorded in sinusoids and THVs, ie, completely stagnant blood flow, intermittent and slow flow, normal flow.
Twenty minutes (the earliest time) after injection of DSM, small pieces of debris could be found in some sinusoids. Some of the debris, with a relatively small size, could directly pass through the sinusoids and flow into THVs. Some debris, with larger size, could occlude the corresponding sinusoids. This occlusion was temporary; recanalization could be achieved by opening of collaterals or further distal movement of the disaggregated debris. The number of DSM debris reached a peak value 1 h after DSM injection. No DSM particles with the original size and shape could be found in sinusoids and THVs. Numerous disaggregated debris with small diameter entered the THVs.
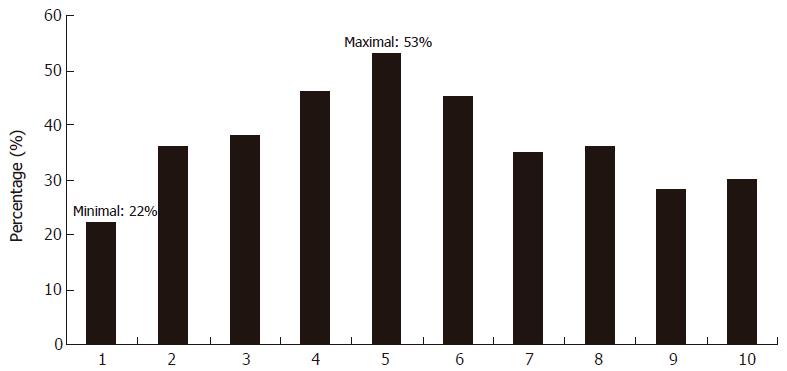
Six hours after DSM injection, the brightness of the liver surface was not uniform after infusion of fluorescent sodium through the femoral vein, suggesting the heterogeneous nature of the liver blood flow. Areas with completely stagnant blood flow in TPVs, THVs and sinusoids could be found sparsely distributed among the areas with normal or sluggish blood flow. Approximately 36.9% ± 9.2% of randomly selected THVs were found with completely stagnant blood flow (Figure 4).
DSM is a kind of embolization material that can temporarily occlude the vessels. The degradation of DSM is considered to be caused by a combination of the chemical effects of amylase and the striking force of the vortexial arterial flow. Hepatic arterial perfusion is essential for an optimal sinusoidal function because it maintains transinusoidal pressure[13]. After intra-artery injection of DSM, immediately slow down or even stop of the blood flow in TPVs, sinusoids, and THVs could be found. It is considered that a sudden reduction of arterial blood flow is caused by numerous DSM casts embolization. The blocked blood flow could soon be resumed due to a compensatory increase of portal blood flow as a buffer response. This can explain the phenomenon of phase one which happened after the DSM injection. After a complete recovery of blood flow in phase one, presinusoidal A-P shunt[14-16] should be the reason for the appearance of three types of blood flow in phase two. We have found in our previous study that after intra-arterial injection of DSM, various sizes of DSM casts are formed inside the arterioles which can block the arterial blood flow. The proximal end of DSM casts will disaggregate under the pumping force of vortexial arterial blood flow. Debris with different sizes will be discharged at the proximal end and further occlude the branch of the original artery. We presume that A-P shunts have various size, some larger debris of the disaggregated DSM can pass through larger A-P shunts and reach the portal side that is proximal to TPVs. Debris accumulated at the portal side form a number of emboli. These emboli, if big enough, will completely shut the portal blood flow to distal TPVs and be harder to disaggregate because the pumping force of portal blood stream is much weaker than that of the arterial one. This means, at certain areas of liver parenchyma, both arterial and portal blood flows are stopped by DSM casts and the debris emboli. Few debris could be found in the distal TPVs because the proximal portion of TPVs was completely occluded by the debris emboli. This explains the type one phenomenon. For some small A-P shunt, only small-sized debris can pass through; and these smaller debris could partially occlude portal branch and was easily to be pushed distally. Some could reach TPVs and flow through sinusoids to THVs. This caused type two phenomenon. As for the type three phenomenon, it is considered that no debris of DSM entered portal site through A-P shunt.
After entering sinusoids, debris could occlude sinusoids. For a single sinusoid, the blood flow can be resumed either by further disaggregation of the debris or by opening of the small collaterals. Small DSM debris, when passing through sinusoid, will flow freely into THVs. Six hours after injection of DSM, we found 22%-53% (mean: 36.9% ± 9.2%) of THVs with totally stagnant blood flow. That means all sinusoids draining blood to these THVs had been stagnant in blood flow. The corresponding liver parenchyma received no fresh blood supply during this time. The cause of stagnant blood flow in sinusoids surrounding THVs is presumed at the presinusoidal level. We assume that the occlusion site was the presinusoidal portal vein with relatively larger diameter. After arterial embolization by DSM casts, the more bigger debris of DSM entered this portion through larger A-P shunt and accumulation of these debris formed intravascular emboli. Weak pumping force of portal blood flow could not disaggregate these emboli, and the amylase could take effects more slowly because little fresh blood flow could reach those emboli. With all those factors, the emboli could maintain stable during a fairly long period of time. Thus, a simultaneous blockade of the arterial and portal blood flow could lead to a completely stagnant blood flow in distal sinusoids. Because the amylase in blood flow will chemically disaggregate the DSM and its debris, whether the TPVs, sinusoids and THVs can resume their blood flow later needs to be further studied.
It is preliminarily confirmed in this study that DSM, with its degradation products, can enter portal vein through hepatic arterial injection. It can completely stop the microcirculatory blood flow in some areas of liver parenchyma. A-P shunt is considered to be a determining factor during the procedure. Liver parenchyma supplied by arteries with larger A-P shunt is presumed to have higher risk of total microcirculatory blood stagnation after injection of DSM through hepatic artery. Whether the use of DSM can provide protective effects during TACE awaits further evaluation.
S- Editor Pan BR L- Editor Zhu LH E- Editor Bi L
| 1. | Kim P, Prapong W, Sze DY, So SK, Razavi MK. Treatment of hepatocellular carcinoma with sub-selective transcatheter arterial oily chemoinfusion. Tech Vasc Interv Radiol. 2002;5:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 1987] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 3. | Chen MS, Li JQ, Zhang YQ, Lu LX, Zhang WZ, Yuan YF, Guo YP, Lin XJ, Li GH. High-dose iodized oil transcatheter arterial chemoembolization for patients with large hepatocellular carcinoma. World J Gastroenterol. 2002;8:74-78. [PubMed] |
| 4. | Bhattacharya S, Dusheiko GM. Treatment of unresectable hepatocellular carcinoma: targeted therapies using iodized oil. Princess Takamatsu Symp. 1995;25:253-264. [PubMed] |
| 5. | Kan Z, Sato M, Ivancev K, Uchida B, Hedgpeth P, Lunderquist A, Rosch J, Yamada R. Distribution and effect of iodized poppyseed oil in the liver after hepatic artery embolization: experimental study in several animal species. Radiology. 1993;186:861-866. [PubMed] |
| 6. | Kan Z, Wallace S. Sinusoidal embolization: impact of iodized oil on hepatic microcirculation. J Vasc Interv Radiol. 1994;5:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Kan Z, Ivancev K, Hägerstrand I, Chuang VP, Lunderquist A. In vivo microscopy of the liver after injection of Lipiodol into the hepatic artery and portal vein in the rat. Acta Radiol. 1989;30:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Akashi Y, Koreeda C, Mizuno T, Inoue K, Kawa SK, Tanaka Y. Hepatic parenchymal changes after the intraarterial injection of lipiodol in tumor-bearing rabbits. Invest Radiol. 1993;28:128-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Sato M, Yamada R, Uchida B, Hedgepeth P, Rosch J. Effects of hepatic artery embolization with Lipiodol and gelatin sponge particles on normal swine liver. Cardiovasc Intervent Radiol. 1993;16:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Furuse J, Ishii H, Satake M, Onaya H, Nose H, Mikami S, Sakai H, Mera K, Maru Y, Yoshino M. Pilot study of transcatheter arterial chemoembolization with degradable starch microspheres in patients with hepatocellular carcinoma. Am J Clin Oncol. 2003;26:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 11. | Kigami Y, Kobayashi H, Umeoka S, Emoto T, Akuta K. [Early effect of intra-arterial chemotherapy combined with degradable starch microspheres for malignant hepatic tumors]. Gan To Kagaku Ryoho. 2003;30:81-87. [PubMed] |
| 12. | Katsumata K, Tomioka H, Sumi T, Yamasaki T, Takagi M, Kato F, Suzuki Y, Aoki T, Koyanagi Y. Liver metastasis of pancreatic cancer managed by intra-arterial infusion chemotherapy combined with degradable starch microspheres. Int J Clin Oncol. 2003;8:110-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Alexander B, Cottam H, Naftalin R. Hepatic arterial perfusion regulates portal venous flow between hepatic sinusoids and intrahepatic shunts in the normal rat liver in vitro. Pflugers Arch. 2001;443:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Grisham JW, Nopanitaya W. Scanning electron microscopy of casts of hepatic microvessels: review of methods and results. Hepatic circulation in health and disease. New York: Raven Press 1981; 87-109. |
| 15. | McCuskey RS. A dynamic and static study of hepatic arterioles and hepatic sphincters. Am J Anat. 1966;119:455-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 156] [Article Influence: 2.6] [Reference Citation Analysis (0)] |