Published online Jul 7, 2006. doi: 10.3748/wjg.v12.i25.4101
Revised: January 10, 2006
Accepted: January 14, 2006
Published online: July 7, 2006
We here report a case of a 51-year-old man with lung metastasis from esophageal carcinoma that was initially treated by combination chemotherapy consisting of fluorouracil and nedaplatin. Because metastatic disease disappeared, salvage esophagectomy was performed. Although chest wall recurrence developed at the thoracotomy wound, prolonged survival of 48 months was achieved by local tumor resection and additional chemotherapy. This combination chemotherapy is regarded as a promising and considerable treatment for metastatic esophageal carcinoma.
- Citation: Kosugi SI, Kanda T, Nishimaki T, Nakagawa S, Yajima K, Ohashi M, Hatakeyama K. Successful treatment for esophageal carcinoma with lung metastasis by induction chemotherapy followed by salvage esophagectomy: Report of a case. World J Gastroenterol 2006; 12(25): 4101-4103
- URL: https://www.wjgnet.com/1007-9327/full/v12/i25/4101.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i25.4101
It has been reported that 18% of patients with newly diagnosed esophageal carcinoma have distant metastatic disease at presentation, in which the lung is the third most common site and accounts for 20% of metastasis cases[1]. The prognosis of these patients is extremely poor and the urgent establishment of effective treatment is essential. We here describe a successful treatment for esophageal carcinoma with lung metastasis by induction chemotherapy followed by salvage esophagectomy.
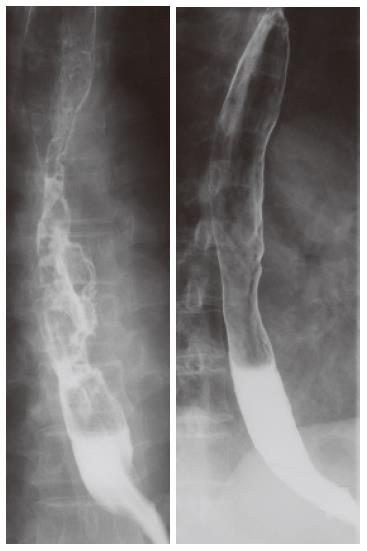
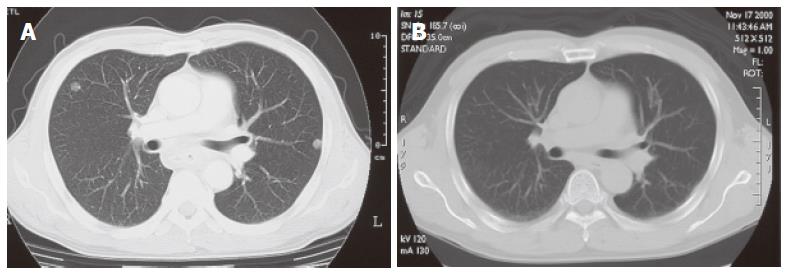
A 51-year-old man complained of dysphagia for over two months. Endoscopy and a computed tomography (CT) scan of the chest performed in an affiliated hospital showed a circumferential tumor in the middle thoracic esophagus with bilateral multiple pulmonary nodules. He was referred to our hospital for further examination and treatment, and admitted on May 24, 2000. On physical examination, lymph nodes were palpable in the left supraclavicular fossa. Laboratory data including tumor markers were all within normal limits. A chest X-ray showed multiple small nodules in the bilateral lung. A barium meal study revealed an ulcerative tumor, 20 cm in length, mainly located in the middle and lower thoracic esophagus extending to the upper thoracic esophagus (Figure 1A). Endoscopy disclosed a protruding tumor 25 cm distal from the dental arch followed by a circumferential ulcerative tumor between 30 cm and 38 cm distal from the dental arch. A biopsy specimen histologically proved that it was a moderately differentiated squamous cell carcinoma. A CT scan of the neck through the abdomen demonstrated bilateral supraclavicular lymph node swelling and a total of 8 small nodules in the bilateral lung up to 1 cm in size with clear boundary (Figure 2A). Based on these findings, he was diagnosed with unresectable thoracic esophageal carcinoma with metastases to non-regional lymph nodes and lung, classified as stage IVB according to the TNM classification of the International Union Against Cancer (UICC)[2]. With his written informed consent, he was registered with the Japan Clinical Oncology Group (JCOG) Trial (JCOG 9905), which was a phase II study of nedaplatin (NDP) and 5-fluorouracil (5-FU) in metastatic squamous cell carcinoma of the esophagus. Each course of chemotherapy consisted of 140 mg of NDP on d 1 and 1250 mg of 5-FU with continuous infusion on d 1-5. Courses were repeated every 4 wk, and a total of 4 courses were performed. Hematological and non-hematological toxicities that developed during chemotherapy were grade 1 leukocytopenia and grade 3 anorexia, nausea, and stomatitis, respectively, as judged by the Common Toxicity Criteria of the National Cancer Institute (NCI-CTC) Version 2.0. After the completion of the 4th course of this combined chemotherapy, a CT scan revealed that multiple nodules in the bilateral lung disappeared (Figure 2B). No lymph node swelling was seen on the CT scan. On barium meal study, an irregular and slightly stenotic lesion, only 4 cm in length, was demonstrated in the middle thoracic esophagus (Figure 1B). A residual esophageal tumor was, however, endoscopically apparent as a slightly depressed lesion with a Lugol unstained area, which was histologically confirmed. Salvage esophagectomy was taken into consideration because the combined chemotherapy downstaged the patient’s condition to that of a resectable disease.
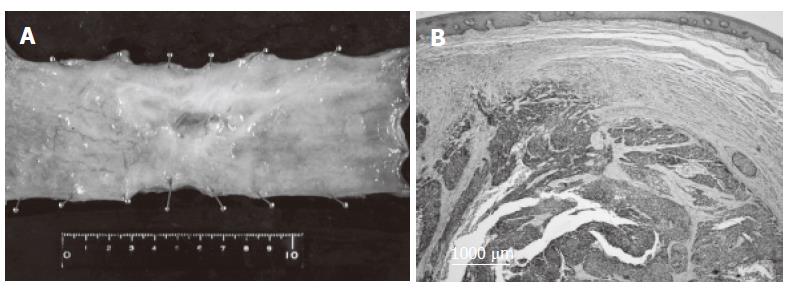
On November 20, 2000, the patient underwent extended radical esophagectomy with a three-field lymphadenectomy, and reconstruction with a retrosternal gastric roll. No metastatic lesion was macroscopically found in the right lung during surgery. The resected specimen showed that the superficially invasive tumor was 78 mm × 60 mm in size (Figure 3A). Histological examination from the resected specimen showed foci of poorly-differentiated squamous cell carcinoma scattered in the submucosal layer (Figure 3B). No metastatic node was histologically identified. The histopathological response to chemotherapy was classified as grade 2 (moderately effective) according to the guidelines of the Japanese Society for Esophageal Diseases[3]. Consequently, R0 resection, according to the residual tumor classification of the UICC-TNM classification, was carried out[2].
The patient had an uneventful postoperative course and was discharged 26 d after surgery. He was followed up at our outpatient department without any additional adjuvant therapy.
Chest wall recurrence probably due to tumor cell implantation developed at the thoracotomy wound nine months after esophagectomy. Because no other recurrence was detected, tumor resection was performed for local control. R1 resection was confirmed, as positive involvement of poorly-differentiated squamous cell carcinoma with the external surface of the resected specimen was histologically proved. Thereafter, the patient received two cycles of additional combined chemotherapy consisting of NDP and 5-FU, and was alive and well with no evidence of recurrence 48 mo after the second surgery.
The prognosis of patients with stage IVB esophageal carcinoma is extremely dismal. The 5-year survival rate of such patients after esophagectomy reported by the Japanese Society for Esophageal Diseases is 5.5%[4]. The stage IVB disease comprises distinct two subgroups: one with distant organ metastasis and the other with non-regional nodal metastasis[2]. Because esophagectomy has been mainly performed in the latter, overall survival of the disease is supposed to be even worse. Kato et al[5] reported that the 3-year survival rate for esophageal cancer patients with distant organ metastasis is only 0.3%. Patients with distant organ metastasis are usually in poor condition at the time of presentation and receive only palliative treatment, radical treatment is selected only for patients in relatively good condition.
Surgery should be taken into consideration when complete resection of both primary and metastatic tumors is possible. In many cases, however, complete resection is impossible because multiple metastatic tumors are often detected in one or more organs. Although there are some reports on successful resection of esophageal carcinoma combined with liver metastasis[6,7] or adrenal metastasis [8], these cases seem to be exceptional.
Chemoradiotherapy is often performed in patients with non-regional nodal metastasis by extending the irradiation field[9,10]. Nakada et al[11] reported that complete regression of esophageal cancer with concomitant liver metastasis and a long-term survival of 38 mo is achieved by concurrent chemoradiotherapy including metastatic lesions in the irradiation field. Except for these unusual cases, chemoradiotherapy is regarded as locoregional treatment in principle and should not be radical treatment for patients with distant organ metastasis.
Systemic chemotherapy is still widely accepted as a standard treatment for patients with distant organ metastasis. It is well known that squamous cell carcinoma of the esophagus is responsive to chemotherapy. Tumor reduction by at least 50% may occur in 35%-55% of patients with metastatic disease who receive cisplatin (CDDP)-based combination chemotherapy[12]. Although chemotherapy can palliate symptoms in many patients, the response to chemotherapy typically lasts no longer than a few months and the median survival time of these patients is less than one year. To our knowledge, there is no other case in which an esophageal cancer patient with stage IV disease is down-staged by systemic chemotherapy, undergoes salvage esophagectomy for the residual esophageal tumor, and survives more than 4 years.
NDP is a second-generation platinum complex that has a characteristic property of being approximately 10 times as soluble in water as CDDP. Therefore, NDP is considered to have more pronounced activity against solid tumors and less nephrotoxicity and gastrointestinal toxicity than CDDP[13]. A phase II study for metastatic squamous cell carcinoma of the esophagus using a combination of NDP and 5-FU has been conducted as a JCOG trial (JCOG 9905) in expectation of preferable anti-tumor effects and toxicities compared with that of CDDP and 5-FU[14]. As a result, the overall response rate is 39.5% and 33.3% in metastatic lung disease[14]. Based on these results and the current case, combined chemotherapy consisting NDP and 5-FU is regarded as a promising and considerable treatment for metastatic squamous cell carcinoma of the esophagus.
S- Editor Wang J L- Editor Wang XL E- Editor Liu Y
| 1. | Quint LE, Hepburn LM, Francis IR, Whyte RI, Orringer MB. Incidence and distribution of distant metastases from newly diagnosed esophageal carcinoma. Cancer. 1995;76:1120-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 2. | Sobin LH, Wittekind C, eds . TNM classification of malignant tumours, 5th ed. New York: Wiley 1997; . |
| 3. | Japanese Society for Esophageal Disease. Guidelines for the clinical and pathological studies on carcinoma of the esophagus, 9th ed. Japan: Kanehara 1999; . |
| 4. | Japanese Society for Esophageal Diseases. Comprehensive registry of esophageal cancer in Japan, 1999. Esophagus. 2005;2:43-69. [DOI] [Full Text] |
| 5. | Kato H, Tachimori Y, Watanabe H, Iizuka T. Evaluation of the new (1987) TNM classification for thoracic esophageal tumors. Int J Cancer. 1993;53:220-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Hanazaki K, Kuroda T, Wakabayashi M, Sodeyama H, Yokoyama S, Kusama J. Hepatic metastasis from esophageal cancer treated by surgical resection and hepatic arterial infusion chemotherapy. Hepatogastroenterology. 1998;45:201-205. [PubMed] |
| 7. | Yamamoto T, Tachibana M, Kinugasa S, Yoshimura H, Nagasue N. Esophagectomy and hepatic arterial chemotherapy following hepatic resection for esophageal cancer with liver metastasis. J Gastroenterol. 2001;36:560-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Yoshizumi Y, Morisaki Y, Koike H, Shibata H, Yanagawa R, Sugiura Y, Tanaka S. Successful combined resection of carcinoma of the esophagus and adrenal metastasis: report of a case. Surg Today. 1997;27:330-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Ohtsu A, Boku N, Muro K, Chin K, Muto M, Yoshida S, Satake M, Ishikura S, Ogino T, Miyata Y. Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol. 1999;17:2915-2921. [PubMed] |
| 10. | Kobayashi T, Yoshida M, Kawabe A, Isogaki J, Wada H, Kazui T, Nozue M, Nishimura T. [A case of stage IV esophageal cancer successfully treated by chemoradiation]. Gan To Kagaku Ryoho. 1999;26:2233-2236. [PubMed] |
| 11. | Nakada T, Nagayama K, Hiramoto J, Tsuruta Y, Murakami S, Sakabe S. Complete regression of esophageal cancer with concomitant liver metastasis achieved by concurrent chemoradiation therapy. Int J Clin Oncol. 2002;7:192-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2219] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 13. | Kosugi S, Kanda T, Nakagawa S, Ohashi M, Nishimaki T, Hatakeyama K. Efficacy and toxicity of fluorouracil, doxorubicin, and cisplatin/nedaplatin treatment as neoadjuvant chemotherapy for advanced esophageal carcinoma. Scand J Gastroenterol. 2005;40:886-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Muro K. A phase II study of nedaplatin and 5-fluorouracil in metastatic squamous cell carcinoma of the esophagus: The Japan Clinical Oncology Group (JCOG) Trial (JCOG 9905). Gan No Rinsho. 2004;50:269-275. |