Published online Jun 7, 2006. doi: 10.3748/wjg.v12.i21.3338
Revised: November 28, 2005
Accepted: February 18, 2006
Published online: June 7, 2006
AIM: To evaluate prospectively 4 selected serum fibrosis markers (tenascin, hyaluronan, collagen VI, TIMP-1) before, during and 12 mo after IFN treatment of children with chronic hepatitis B.
METHODS: Forty-seven consecutive patients with chronic hepatitis B (range 4-16 years, mean 8 years) underwent IFN treatment (3 MU tiw for 20 wk). Fibrosis stage and inflammation grade were assessed in a blinded fashion before and 12 mo after end of treatment. Serum fibrosis markers were determined using automated assays.
RESULTS: IFN treatment improved histological inflammation but did not change fibrosis in the whole group or in subgroups. Only hyaluronan correlated significantly with histological fibrosis(r = 0.3383, P = 0.021). Basal fibrosis markers did not differ between responders (42.5%) and nonresponders(57.5%). During IFN treatment only serum tenascin decreased significantly in the whole group and in nonresponders. When pretreatment values were compared to values 12 mo after therapy, TIMP-1 increased in all patients and in nonresponders, and hyaluronan decreased in all patients and in responders.
CONCLUSION: Tenascin reflects hepatic fibrogenesis and inflammation which decreases during IFN treatment of children with chronic hepatitis B. TIMP-1 correlates with nonresponse and hyaluronan with histological fibrosis.
- Citation: Lebensztejn DM, Sobaniec-Lotowska ME, Kaczmarski M, Voelker M, Schuppan D. Matrix-derived serum markers in monitoring liver fibrosis in children with chronic hepatitis B treated with interferon alpha. World J Gastroenterol 2006; 12(21): 3338-3343
- URL: https://www.wjgnet.com/1007-9327/full/v12/i21/3338.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i21.3338
The potential of interferon α (IFNα) to normalize aminotransferase activity, eliminate serum HBV DNA and HBeAg and reduce liver necroinflammation in patients with chronic hepatitis B is widely acknowledged[1-3]. In children with chronic hepatitis B treatment with IFN leads to long-term serological and biochemical remission in less than 50%[4-6]. Nonetheless, several reports suggested that IFN treatment for hepatitic C can halt or even reverse liver fibrosis[7-10], while its antifibrogenic potential in chronic hepatitis B needs to be confirmed[11]. Histological staging of liver fibrosis plays a central role in the liver pathological assessment since progressive fibrosis may lead to cirrhosis, the most important predictor of decompensated liver diseases and death[3,12].
Liver biopsy is the standard method to assess fibrosis stage which allows to semiquantify the extent of fibrosis, yielding a static view[13]. However, biopsy has significant disadvantages for the assessment of fibrosis and fibrosis progression. Thus despite minor histological fibrosis, progression might be fast, since the accumulated extracellular matrix is the result of a dynamic process characterized by changes in matrix synthesis (fibrogenesis) and removal (fibrolysis)[14]. Furthermore, liver biopsy is invasive and histological scoring systems are not sensitive enough to detect small changes in fibrosis stage. Finally, bioptical sampling error can reach 25%-33% for a difference in one stage, when using the METAVIR system which ranges from 0 (no fibrosis) to 4 (cirrhosis)[15,16]. Therefore, noninvasive markers that may reflect overall hepatic fibrogenesis and fibrolysis in chronic hepatitis would be of great clinical benefit, allowing repeated assessment of progression or therapeutic interventions, especially in children with chronic hepatitis B or C who are treated with IFN or other antiviral or potential antifibrotic agents[17-19].
The aim of this study was to investigate the clinical usefulness of selected matrix-derived serum markers (tenascin, hyaluronan, collagen VI, tissue inhibitor of metalloproteinase 1 or TIMP-1) in a long-term follow-up of children with chronic hepatitis B treated with IFN α.
The study was carried out prospectively in 47 children (mean age 8 years, range 4-16, 31 boys and 16 girls) with serologically and biopsy-verified chronic hepatitis B. The children were positive for HBs and HBe antigens and had increased serum activity of HBV DNA polymerase for at least 1 year. Patients with autoimmune hepatitis or HCV coinfection were excluded from the study. None of the children was treated with antiviral and immunomodulating drugs during the 12-month period before inclusion into the study. Informed consent was obtained from all patients’ parents and the protocol was approved by the local ethical committee of the Medical University of Bialystok. Serum samples were evaluated at three time points: at the start and the end (5 mo) of IFN α treatment, and 12 mo after end of treatment. Serum samples were stored at -70 °C until use. Standard liver tests were measured by validated automated methods and included total bilirubin, albumin, alanine transaminase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (gamma-GT). HBsAg and HBeAg were determined by MEIA (IMx, Abbott).
IFN (IFN α 2a: 30 children or IFN α 2b: 17 children) was applied at the dose of 3 MU tiw subcutaneously for 20 wk according to the schedule approved by the Polish Interferon Study Group[20]. HBeAg/antiHBe seroconversion and lack of HBV DNA polymerase activity 1 year after end of treatment was considered as the criterion of treatment response.
Monoclonal antibodies were used to detect tenascin, collagen VI and TIMP-1 in sandwich immunoassays performed in an automated analyzer employing fluoresceine-labelled capture antibodies and alkaline phosphatase labeled detection antibodies. Hyaluronan was determined using biotinylated cartilage link protein. The immune complexes were separated from serum using magnetic particles covered with monoclonal anti-fluoresceine (anti-biotin in case of hyaluronan). The assays were developed for the BAYER IMMUNO 1 immunoassay system and validated in several cohorts of liver patients and healthy individuals[21-23].
Percutaneous liver biopsies were obtained before treatment and 12 mo after IFN α discontinuation. The liver specimens were fixed in buffered formalin and embedded in paraffin. Histological sections were stained using hematoxylin-eosin, Masson-Goldner, Masson’s trichrome and reticulin stains. Fibrosis stage and inflammation grade were assessed in a blinded fashion by a single pathologist according to the method of Batts and Ludwig[24].
Results were expressed as means ± SD. Statistical analysis was performed with the Wilcoxon rank-sum test for independent samples and Wilcoxon signed rank test for paired samples. The relationship between the serum fibrosis markers and liver histology scores was analysed by the Spearman rank-correlation test for nonparametric data and by the Pearson method for parametric data. Tests were considered statistically significant at P < 0.05.
The baseline characteristics of the 47 children are presented in Table 1. They were classified into responders (n = 20; 42.5%) and nonresponders (n = 27; 57.5%). There were no significant differences between the groups regarding age, gender, body mass, duration of HBV infection, the levels of bilirubin and albumin, the activity of GGT, levels of baseline liver fibrosis markers (tenascin, hyaluronan, collagen VI, TIMP-1) and grade of inflammation. However, treatment responders displayed a significantly higher activity of ALT (2184 ± 2217 vs 1150 ± 550 nkat/L) and AST (1717 ± 1567 vs 1100 ± 500 nkat/L) (P = 0.0453 for both) and a higher fibrosis score according to Batts and Ludwig (2.3 ± 0.5 vs 1.8 ± 0.6; P = 0.004).
| Data of the patients | Mean | SD | Minimum | Maximum |
| Age (yr) | 8 | 3.51 | 4 | 16 |
| HBV infection (mo) | 44 | 30 | 10 | 144 |
| ALT (nkat/L) | 1600 | 1567 | 350 | 9902 |
| AST (nkat/L) | 1367 | 1117 | 567 | 7718 |
| GGT (nkat/L) | 250 | 150 | 50 | 1150 |
| Bilirubin (μmol/L) | 8.5 | 4.3 | 3.4 | 23.9 |
| Albumin (g/L) | 65.6 | 4.2 | 57.2 | 74.3 |
| Hyaluronan (μg/L) | 34.3 | 21.5 | 14.6 | 113.5 |
| Tenascin (μg/L) | 748.8 | 265.9 | 295.6 | 1480.2 |
| TIMP-1(μg/L) | 558.1 | 121.2 | 342.2 | 852.3 |
| Collagen VI (μg/L) | 5.8 | 2.2 | 2.3 | 14.2 |
| Staging | 2.0 | 0.6 | 1 | 3 |
| Grading | 1.6 | 0.7 | 1 | 3 |
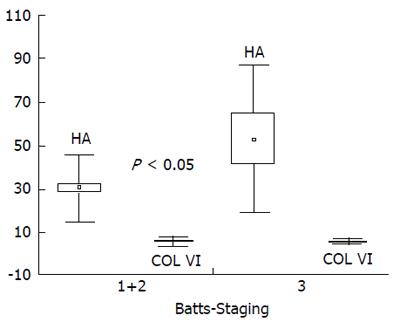
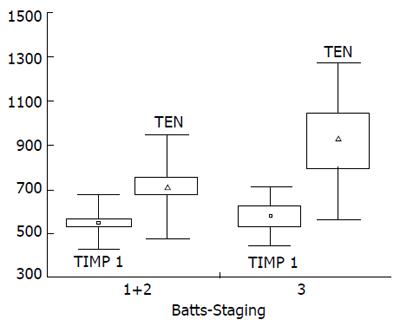
We arbitrarily defined mild/moderate fibrosis as stage ≤ 2 (n = 39) and advanced fibrosis as stage = 3 (n = 8) according to Batts and Ludwig. There were no significant differences in mean serum levels of tenascin, collagen VI and TIMP-1 between children with mild and advanced liver fibrosis, while levels of hyaluronan were higher in the latter group (30.3±15.8 vs 53.1±34.3 μg/L; P = 0.0266). There was a trend for increased tenascin and TIMP-1 in children with advanced fibrosis (Figure 1, Figure 2). We also arbitrarily defined mild inflammation as grade 1 (n = 20) and severe inflammation as grade ≥2 (n = 27) according to Batts and Ludwig. There were no significant differences in mean concentrations of all serum fibrosis markers between children with mild and severe hepatic inflammation.
At end of treatment there were no significant changes in serum fibrosis markers in responders, while in nonresponders only tenascin decreased significantly (P = 0.0074). Twelve months after end of treatment serum hyaluronan was significantly lower than before treatment (P = 0.0076), while serum TIMP-1 was increased (P = 0.0072). In responders only hyaluronan decreased significantly (P = 0.0304), while in nonresponders the level of TIMP-1 increased (P = 0.0064, Table 2). Tenascin reached pretreatment levels in both responders and nonresponders.
| Patients | Marker(μg/L) | Before IFN(1) | After IFN(2) | 12 mo after IFN (3) | P1 vs 2 | P1 vs 3 | P2 vs 3 |
| All n = 47 | TIMP-1 | 558.1 ± 121.2 | 569.2 ± 134.8 | 636.9 ± 125.8 | NS | b | NS |
| CollagenVI | 5.8 ± 2.2 | 6.2 ± 1.8 | Nd | NS | - | - | |
| Tenascin | 748.8 ± 266.0 | 641.5 ± 216.8 | 764.0 ± 250.0 | b | NS | b | |
| Hyaluronan | 34.3 ± 21.5 | 40.0 ± 30.9 | 28.7 ± 19.3 | NS | b | b | |
| Responders n = 20 | TIMP-1 | 544.1 ± 120.3 | 555.4 ± 109.3 | 608.0 ± 134.9 | NS | NS | NS |
| CollagenVI | 5.6 ± 1.85 | 6.7 ± 1.8 | Nd | NS | - | - | |
| Tenascin | 766.2 ± 289.9 | 686.2 ± 225.8 | 765.5 ± 249.9 | NS | NS | NS | |
| Hyaluronan | 40.3 ± 28.1 | 36.8 ± 18.0 | 30.7 ± 17.2 | NS | a | a | |
| Non Responders n = 27 | TIMP-1 | 567.9 ± 123.1 | 581.2 ± 155.0 | 657.2 ± 118.3 | NS | b | NS |
| CollagenVI | 6.0 ± 2.4 | 5.7 ± 1.7 | Nd | NS | - | - | |
| Tenascin | 736.6 ± 252.7 | 602.6 ± 205.6 | 762.9 ± 254.9 | b | NS | b | |
| Hyaluronan | 29.6 ± 13.6 | 42.7 ± 39.1 | 27.2 ± 20.9 | NS | NS | b |
There were no significant changes in fibrosis stage after IFN therapy in the whole cohort, 2.0 ± 0.6 vs 2.1 ± 0.6, according to Batts and Ludwig and in subgroups, responders: 2.3 ± 0.5 vs 2.2 ± 0.7; nonresponders: 1.8 ± 0.6 vs 2.0 ± 0.6. Histological inflammation improved significantly in the whole group, 1.6 ± 0.7 vs 1.2 ± 0.7, P = 0.0373.
There were no significant correlations between baseline levels of the 4 serum fibrosis markers with liver fibrosis or inflammation according to Batts and Ludwig, or with AST, ALT, GGT, albumin or bilirubin. Only hyaluronan correlated significantly with histological fibrosis (r = 0.3383, P = 0.021).
Liver biopsy has been considered the gold standard for the assessment of hepatic fibrosis. Current recommendations suggest that this procedure precede antiviral treatment in most patients with chronic hepatitits B or C[25]. However, liver biopsy is invasive with the potential for complications, such as bleeding which occurrence ranges from 0.3% to 0.5%[26,27], and mortality up to 0.1%[26,28]. In addition, since the biopsy core only represents 1/20 000 to 1/50 000 of the liver, biopsy is prone to sampling error, and variations in fibrosis staging may be high among different pathologists[15,16,21,29]. For these reasons, especially in children, non-invasive detection of histological liver damage, paticularly of fibrosis, is needed. Ideally serum markers of fibrosis should be applicable to patients with chronic hepatitis to either diagnose the stage of liver fibrosis, potentially replacing liver biopsy for this purpose, or to monitor progression of fibrosis or fibrogenesis, particulary during treatment [17,30]. Markers of the dynamics of fibrogenesis and fibrolysis are urgently needed, e.g. for short-term assessment of antifibrotic drug effects, but difficult to validate.
In this study we evaluated the changes of 4 serum fibrosis markers derived from the extracellular matrix (tenascin, hyaluronan, collagen VI and TIMP-1) before, at the end of and 12 mo after treatment of children with chronic hepatitis B with IFN. Our results showed a significant decrease of hyaluronan in responders and increased TIMP-1 in nonresponders, when levels before and 12 mo after interferon α treatment were compared. While falling during treatment, serum tenascin reached pretreatment levels in both responders and nonresponders. There were no significant changes in histological liver fibrosis 12 mo after the 5-mo course of IFN in all patients or in the subgroups of responders and nonresponders. This was expected, since the rate of fibrosis progression or regression in patients with chronic hepatitis B or C was usually slow. Assuming that IFN has at least some antifibrotic activity, as suggested before in large retrospective analyses of patients with chronic hepatitis C[31,32], the histological scoring systems are obviously not sensitive enough to detect small changes in liver fibrosis and (modest) antifibrotic treatment effects. Nonetheless, the course of serum fibrosis (fibrogenesis) markers in our small but well defined group of children suggests that IFN indeed has antifibrogenic activity, especially in responders. This antifibrotic effect seems to be transient, as exemplified by serum tenascin which was depressed only during IFN treatment.
Prior to our study there had been no longitudinal, prospective studies of serum fibrosis markers in children with chronic hepatitis B, and only few studies analysed the effect of IFN therapy on the stage of liver fibrosis in children. Our findings are consistent with our previous study[33] and with those of others, who did not observe improvement of liver fibrosis by antiviral treatment in children, when biopsy was performed before and immediately after[34] or 9-12 mo after end of treatment[35,36], while Gregorio et al[37] found significant improvement in staging in responders. However, these reports included small numbers of patients (≤24). It has been demonstrated that fibrosis stage changes more slowly than inflammation grade[7,38]. This explains why we did not observe a significant improvement in fibrosis, while inflammation was clearly suppressed by IFN treatment.
We found that hyaluronan was the best serum marker to predict advanced liver fibrosis, since its level correlated significantly with histological fibrosis and was significantly higher in children with advanced vs mild/moderate liver fibrosis. These data are in keeping with previous results in patients with chronic viral hepatitis[39-43]. Thus the ability of this test to differentiate patients with extensive liver fibrosis from those with mild liver fibrosis was stronger than that of other markers, i.e., PIIINP, collagen IV, MMP-1, MMP-2 and TIMP-1[44] and laminin, collagen IV, PIIINP and TGF β1[45].
In children, up to now serum hyaluronan had only been studied in biliary atresia and cystic fibrosis[46-48], and there had been no data on collagen VI in chronic viral hepatitis or on TIMP-1 or tenascin in childhood liver diseases in general. Previous studies indicated that most serum fibrosis markers are influenced by body growth, especially PIIINP[49,50]. Thus healthy children have higher PIIINP levels than adults, excluding its use as reliable fibrosis marker for pediatric patients. Hyaluronan appears to be a useful marker of fibrosis stage also in children due to its short biological half life of only a few minutes and a prominent uptake by sinusoidal endothelial cells[51]. Similarly, tenascin and TIMP-1 are applicable to children with chronic hepatitis B as markers of fibrogenesis/inflammation and of fibrogenesis, respectively.
Our data suggest that serum hyaluronan, tenascin and TIMP-1 could be useful fibrosis markers in future studies of children with chronic viral hepatitis.
S- Editor Pan BR L- Editor Zhu LH E- Editor Zhang Y
| 1. | Wong DK, Cheung AM, O'Rourke K, Naylor CD, Detsky AS, Heathcote J. Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B. A meta-analysis. Ann Intern Med. 1993;119:312-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 705] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 2. | Janssen HL, Gerken G, Carreño V, Marcellin P, Naoumov NV, Craxi A, Ring-Larsen H, Kitis G, van Hattum J, de Vries RA. Interferon alfa for chronic hepatitis B infection: increased efficacy of prolonged treatment. The European Concerted Action on Viral Hepatitis (EUROHEP). Hepatology. 1999;30:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 97] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000--summary of a workshop. Gastroenterology. 2001;120:1828-1853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 513] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 4. | Vajro P, Migliaro F, Fontanella A, Orso G. Interferon: a meta-analysis of published studies in pediatric chronic hepatitis B. Acta Gastroenterol Belg. 1998;61:219-223. [PubMed] |
| 5. | Sokal EM, Conjeevaram HS, Roberts EA, Alvarez F, Bern EM, Goyens P, Rosenthal P, Lachaux A, Shelton M, Sarles J. Interferon alfa therapy for chronic hepatitis B in children: a multinational randomized controlled trial. Gastroenterology. 1998;114:988-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 164] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Bortolotti F, Jara P, Barbera C, Gregorio GV, Vegnente A, Zancan L, Hierro L, Crivellaro C, Vergani GM, Iorio R. Long term effect of alpha interferon in children with chronic hepatitis B. Gut. 2000;46:715-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Shindo M, Arai K, Okuno T. The clinical value of grading and staging scores for predicting a long-term response and evaluating the efficacy of interferon therapy in chronic hepatitis C. J Hepatol. 1997;26:492-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Dufour JF, DeLellis R, Kaplan MM. Regression of hepatic fibrosis in hepatitis C with long-term interferon treatment. Dig Dis Sci. 1998;43:2573-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Bruno S, Battezzati PM, Bellati G, Manzin A, Maggioni M, Crosignani A, Borzio M, Solforosi L, Morabito A, Ideo G. Long-term beneficial effects in sustained responders to interferon-alfa therapy for chronic hepatitis C. J Hepatol. 2001;34:748-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Toccaceli F, Laghi V, Capurso L, Koch M, Sereno S, Scuderi M. Long-term liver histology improvement in patients with chronic hepatitis C and sustained response to interferon. J Viral Hepat. 2003;10:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Han HL, Lang ZW. Changes in serum and histology of patients with chronic hepatitis B after interferon alpha-2b treatment. World J Gastroenterol. 2003;9:117-121. [PubMed] |
| 12. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1712] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 13. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2159] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 14. | Iredale JP. Matrix turnover in fibrogenesis. Hepatogastroenterology. 1996;43:56-71. [PubMed] |
| 15. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1569] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 16. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [PubMed] |
| 17. | Schuppan D, Stölzel U, Oesterling C, Somasundaram R. Serum assays for liver fibrosis. J Hepatol. 1995;22:82-88. [PubMed] |
| 18. | Afdhal NH. Biopsy or biomarkers: is there a gold standard for diagnosis of liver fibrosis. Clin Chem. 2004;50:1299-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 422] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 20. | Woynarowski M, Socha J, Sluzewski W, Chmurska-Motyka T, Mizerski J, Czerwionka-Szaflarska M, Mikina M, Karczewska K, Lebensztejn D, Zaleska I. HBeAg clearance rate and interferon alfa dose. Pediatric Gastroenterology. 2004;253-256. |
| 21. | Ropers T, Kroll W, Becka M, Voelker M, Burchardt ER, Schuppan D, Gehrmann M. Enzyme immunoassay for the measurement of human tenascin-C on the Bayer Immuno 1 analyzer. Clin Biochem. 2000;33:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, Roskams T, Pinzani M, Arthur MJ. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 759] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 23. | Lebensztejn DM, Kaczmarski M, Sobaniec-Łotowska M, Bauer M, Voelker M, Schuppan D. Serum laminin-2 and hyaluronan predict severe liver fibrosis in children with chronic hepatitis B. Hepatology. 2004;39:868-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 847] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 25. | Perrillo RP. The role of liver biopsy in hepatitis C. Hepatology. 1997;26:57S-61S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 101] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology. 2000;32:477-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 731] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 27. | Froehlich F, Lamy O, Fried M, Gonvers JJ. Practice and complications of liver biopsy. Results of a nationwide survey in Switzerland. Dig Dis Sci. 1993;38:1480-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 102] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 28. | McGill DB, Rakela J, Zinsmeister AR, Ott BJ. A 21-year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology. 1990;99:1396-1400. [PubMed] |
| 29. | Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15-20. [PubMed] |
| 30. | Trinchet JC. Clinical use of serum markers of fibrosis in chronic hepatitis. J Hepatol. 1995;22:89-95. [PubMed] |
| 31. | Sobesky R, Mathurin P, Charlotte F, Moussalli J, Olivi M, Vidaud M, Ratziu V, Opolon P, Poynard T. Modeling the impact of interferon alfa treatment on liver fibrosis progression in chronic hepatitis C: a dynamic view. The Multivirc Group. Gastroenterology. 1999;116:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 184] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 32. | Shiffman ML, Hofmann CM, Contos MJ, Luketic VA, Sanyal AJ, Sterling RK, Ferreira-Gonzalez A, Mills AS, Garret C. A randomized, controlled trial of maintenance interferon therapy for patients with chronic hepatitis C virus and persistent viremia. Gastroenterology. 1999;117:1164-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 183] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Maria Elzbieta SL, Marek LD. Histological outcome of chronic hepatitis B in children treated with interferon alpha. World J Gastroenterol. 2005;11:7179-7182. [PubMed] |
| 34. | Ozer E, Ozer E, Helvaci M, Yaprak I. Hepatic expression of viral antigens, hepatocytic proliferative activity and histologic changes in liver biopsies of children with chronic hepatitis B after interferon-alpha therapy. Liver. 1999;19:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Ruiz Moreno M, Jimenez J, Porres JC, Bartolomé J, Moreno A, Carreño V. A controlled trial of recombinant interferon-alpha in Caucasian children with chronic hepatitis B. Digestion. 1990;45:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Ruiz-Moreno M, Rua MJ, Molina J, Moraleda G, Moreno A, García-Aguado J, Carreño V. Prospective, randomized controlled trial of interferon-alpha in children with chronic hepatitis B. Hepatology. 1991;13:1035-1039. [PubMed] [DOI] [Full Text] |
| 37. | Gregorio GV, Jara P, Hierro L, Diaz C, de la Vega A, Vegnente A, Iorio R, Bortolotti F, Crivellaro C, Zancan L. Lymphoblastoid interferon alfa with or without steroid pretreatment in children with chronic hepatitis B: a multicenter controlled trial. Hepatology. 1996;23:700-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Ruiz-Moreno M, Otero M, Millán A, Castillo I, Cabrerizo M, Jiménez FJ, Oliva H, Ramon y Cajal S, Carreño V. Clinical and histological outcome after hepatitis B e antigen to antibody seroconversion in children with chronic hepatitis B. Hepatology. 1999;29:572-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Zheng M, Cai WM, Weng HL, Liu RH. ROC curves in evaluation of serum fibrosis indices for hepatic fibrosis. World J Gastroenterol. 2002;8:1073-1076. [PubMed] |
| 40. | Kelleher TB, Mehta SH, Bhaskar R, Sulkowski M, Astemborski J, Thomas DL, Moore RE, Afdhal NH. Prediction of hepatic fibrosis in HIV/HCV co-infected patients using serum fibrosis markers: the SHASTA index. J Hepatol. 2005;43:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 41. | Patel K, Lajoie A, Heaton S, Pianko S, Behling CA, Bylund D, Pockros PJ, Blatt LM, Conrad A, McHutchison JG. Clinical use of hyaluronic acid as a predictor of fibrosis change in hepatitis C. J Gastroenterol Hepatol. 2003;18:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Guéchot J, Serfaty L, Bonnand AM, Chazouillères O, Poupon RE, Poupon R. Prognostic value of serum hyaluronan in patients with compensated HCV cirrhosis. J Hepatol. 2000;32:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Wong VS, Hughes V, Trull A, Wight DG, Petrik J, Alexander GJ. Serum hyaluronic acid is a useful marker of liver fibrosis in chronic hepatitis C virus infection. J Viral Hepat. 1998;5:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Murawaki Y, Ikuta Y, Okamoto K, Koda M, Kawasaki H. Diagnostic value of serum markers of connective tissue turnover for predicting histological staging and grading in patients with chronic hepatitis C. J Gastroenterol. 2001;36:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Luo R, Yang S, Xie J, Zhao Z, He Y, Yao J. [Diagnostic value of five serum markers for liver fibrosis]. Zhonghua Ganzangbing Zazhi. 2001;9:148-150. [PubMed] |
| 46. | Kobayashi H, Horikoshi K, Yamataka A, Yamataka T, Okazaki T, Lane GJ, Miyano T. Hyaluronic acid: a specific prognostic indicator of hepatic damage in biliary atresia. J Pediatr Surg. 1999;34:1791-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Hasegawa T, Sasaki T, Hoki M, Okada A, Mushiake S, Yagi M, Imura K. Measurement of serum hyaluronic acid as a sensitive marker of liver fibrosis in biliary atresia. J Pediatr Surg. 2000;35:1643-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Wyatt HA, Dhawan A, Cheeseman P, Mieli-Vergani G, Price JF. Serum hyaluronic acid concentrations are increased in cystic fibrosis patients with liver disease. Arch Dis Child. 2002;86:190-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Trivedi P, Cheeseman P, Portmann B, Mowat AP. Serum type III procollagen peptide as a non-invasive marker of liver damage during infancy and childhood in extrahepatic biliary atresia, idiopathic hepatitis of infancy and alpha 1 antitrypsin deficiency. Clin Chim Acta. 1986;161:137-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 50. | Danne T, Grüters A, Schuppan D, Quantas N, Enders I, Weber B. Relationship of procollagen type III propeptide-related antigens in serum to somatic growth in healthy children and patients with growth disorders. J Pediatr. 1989;114:257-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |