Published online May 28, 2006. doi: 10.3748/wjg.v12.i20.3229
Revised: April 6, 2006
Accepted: April 16, 2006
Published online: May 28, 2006
Calcium is an essential ion in both marine and terrestrial organisms, where it plays a crucial role in processes ranging from the formation and maintenance of the skeleton to the regulation of neuronal function. The Ca2+ balance is maintained by three organ systems, including the gastrointestinal tract, bone and kidney.
Since first being cloned in 1993 the Ca2+-sensing receptor has been expressed along the entire gastrointestinal tract, until now the exact function is only partly elucidated. As of this date it still remains to be determined if the Ca2+-sensing receptor is involved in calcium handling by the gastrointestinal tract. However, there are few studies showing physiological effects of the Ca2+-sensing receptor on gastric acid secretion and fluid transport in the colon. In addition, polyamines and amino acids have been shown to activate the Ca2+-sensing receptor and also act as allosteric modifiers to signal nutrient availability to intestinal epithelial cells. Activation of the colonic Ca2+-sensing receptor can abrogate cyclic nucleotide-mediated fluid secretion suggesting a role of the receptor in modifying secretory diarrheas like cholera. For many cell types changes in extracellular Ca2+ concentration can switch the cellular behavior from proliferation to terminal differentiation or quiescence. As cancer remains predominantly a disease of disordered balance between proliferation, termination and apoptosis, disruption in the function of the Ca2+-sensing receptor may contribute to the progression of neoplastic disease. Loss of the growth suppressing effects of elevated extracellular Ca2+ have been demonstrated in colon carcinoma, and have been correlated with changes in the level of CaSR expression.
- Citation: Kirchhoff P, Geibel J. Role of calcium and other trace elements in the gastrointestinal physiology. World J Gastroenterol 2006; 12(20): 3229-3236
- URL: https://www.wjgnet.com/1007-9327/full/v12/i20/3229.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i20.3229
The gastrointestinal tract is in charge of handling the complex issues of nutrient, electrolyte and fluid absorption and the secretion of excess electrolytes and fluids. Body calcium homeostasis is regulated by the PTH and vitamin D feedback loop; additionally calcium plays a key role in many other mechanisms like intracellular signaling, cell differentiation, bone metabolism, etc. After identifying the calcium sensing receptor in several different tissues many studies were undertaken to characterize the role of extracellular calcium as a first messenger and the receptor as a calcium sensor of the cell. Ca2+-sensing receptor transcripts and/or protein are expressed in the gastrointestinal tracts of fish[1], birds[2], amphibia[3,4] and mammals[5-10] including the human[5,11,12]. Tracings of the expression of the receptor in the gastrointestinal tract shows that the receptor goes back in evolution at least as far as cartilaginous fish (elasmobranchs), e.g., the dogfish shark[1]. In both cartilaginous and bony fish, the Ca2+-sensing receptor has been shown to be expressed on apical surfaces of stomach and intestine[1]. More recent evidence suggests that the Ca2+-sensing receptor may have evolved in the early marine environment in order to support osmo-adaptation. This notion is supported by the more general expression of the Ca2+-sensing receptor in many other tissues outside the gastrointestinal tract that are involved in mono-and divalent ion transport both into and out of fish that live in a seawater environment that is rich in divalent minerals and sodium chloride[1,13]. This theme of the Ca2+-sensing receptor linking divalent and monovalent metabolism is echoed in mammals (e.g., effects of the receptor on fluid transport by the colon; discussed later in this review).
In the amphibian, Necturus maculosus, Ca2+-sensing receptor expression was detected on the basal surface of gastric epithelial cells[3]. Wherease in contrast the frog stomach, shows expression of the Ca2+-sensing receptor on the apical membranes of acid-secreting oxyntic cells[4]. In the chicken, Gallus domesticus, the receptor was detected in the duodenum[2]. In mammals, a more complete exploration of Ca2+-sensing receptor expression along the gastrointestinal tract has been performed[5-10,12]. Receptor transcripts and/or protein have been detected in: stomach, small intestinal, and colonic mucosal epithelia, as well as the underlying neural plexuses of Meissner and Auerbach. In addition, Ca2+-sensing receptor expression has also been shown in several human intestinal cell lines (T84, HT-29, Caco-2, FET, SW480, MOSER and CBS;[8,14,15] in addition to primary cultures of human gastric mucosa and human parietal cells[11,16,17].
In mammalian stomach, the Ca2+-sensing receptor has been identified on both apical and basolateral membranes of human G-cells (gastrin secreting cells;[16,17]) and mucous secreting cells[11] and on the basolateral membranes of parietal cells[4,6,18]. In small intestine, both apical and basolateral membranes of villus cells express the Ca2+-sensing receptor[7]. In rat colon the receptor is expressed on both apical and basolateral membranes of surface and crypt epithelial cells[5,7]. A similar pattern of Ca2+-sensing receptor immunostaining in rat was observed in both proximal and distal colon[5]. In the human large intestine, Ca2+-sensing receptor has also been identified on both apical and basolateral membranes of crypts as well as in certain enteroendocrine cells at the base of crypts[7,12].
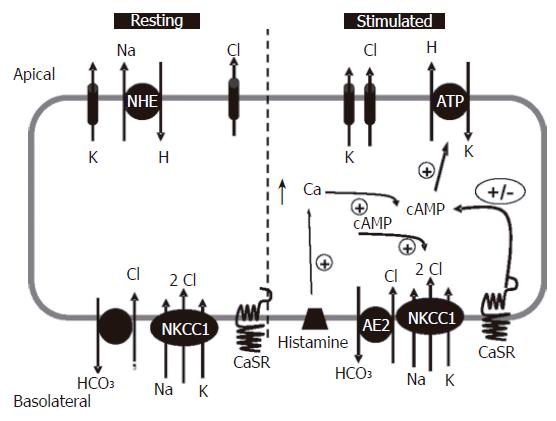
To produce the large quantities of 0.16 mol/L hydrochloric acid required for digestion of ingested food, the mammalian stomach has employed a complex series of neuronal, hormonal and/or paracrine[19]/autocrine feedback regulatory mechanisms[19-22] allowing for the continued production of acid. A model of acid secretion by the parietal cell is shown in Figure 1 summarizing data from many different studies[19,21-24]. Following stimulation, the H+, K+-ATPase (proton pump) is trafficked to the apical surface and is responsible for vectorial transport of protons[20-22,25-27]. Once at the membrane protons combine with secreted Cl- ions to produce the concentrated acid. With the generation of a proton gradient enzymes such as pepsinogen are secreted into the lumen of the gland where they combine with the secreted acid and move from the gland into the interior of the stomach providing an effective solution that is capable of digesting proteins and processing them for amino acid or peptide reabsorption in the intestine.
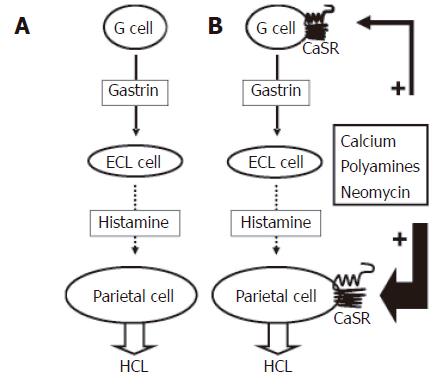
The common model of acid secretion (Figures 1 and 2) involves stimulation of the gastric glands via either neuronal or hormonal pathways which results in the release of gastrin, which in turn acts directly on the endocrine (ECL) cells of the gland. Following stimulation ECL cells secrete histamine causing the parietal cell to insert proton pumps (H+, K+-ATPase) into the apical pole of the gland[28] which occurs via an active tubular-vesicular insertion mechanism that is used to transport H+, K+-ATPases into the apical region of the parietal cell[26]. In conjunction with pump insertion activation of an apical K+ channel(s) provides K+ ions that act as the counter ion and exchange with H+ on the pump[20,21,23,29] (Figure 1) to sustain acid secretion. In addition to cation secretion an additional action of histamine release is to activate and/or insert Cl- channels into the apical membranes of the parietal cells which mediate Cl- secretion that accompanies H+ secretion[20,30,31] (Figure 1). Thus the entire acid secretory process relies on: (1) insertion of H+, K+-ATPase into the apical surface for H+ secretion; (2) concurrent activation of apical Cl- channels mediating Cl- secretion which allows for the formation of HCl; and (3) activation of apical K+ channel(s) for K+ recycling to the lumen of the gland and thereby providing the counter cation K+ to maintain H+, K+-ATPase activity.
Loss of feedback control of acid (HCl) secretion in the stomach causes symptoms ranging from mild heartburn, to lesions and ulcerations of the gastric mucosa[19,32]. Left untreated gastric ulcers can lead to abdominal bleeding, hyperplasia of cells, and potentially tumor formation. The complexity of the tissue due to the mixed collection of cell types, as well an additional layer of surface cells (secreting a protective mucous gel layer rich in bicarbonate) has prevented a complete analysis of glandular cell function and the associated feedback loops.
Following hormonal or neuronal stimulation there is a transient rise in intracellular Ca2+ concurrent with the onset of acid secretion in the gastric gland[19,20,22,33], this process has been associated with activation of pump translocation to the apical pole of the cell , and the associated secretion of acid[34]. Following this stimulatory period intracellular Ca2+ levels fall, and acid secretion diminishes to basal levels.
Activation of basolateral CaSR in Necturus gastric antrum by elevating extracellular Ca2+, or using other receptor agonists like NPS-467, or neomycin resulted in a rapid hyperpolarization and a decrease in resistance of the basolateral membrane. Circuit analysis of these data suggested that these electrophysiological effects were due to activation of a basolateral K+ channel(s)[3]. In rat stomach elevating extracellular Ca2+ leads to a rapid increase in intracellular Ca2+ in parietal cells which can occur in the absence of conventional extracellular secretagogues (e.g. histamine;[6]). To confirm that this rise in Ca2+ was associated with activation of the receptor, studies have been conducted using either the potent agonist Gd3+, or addition of an allosteric modifier of the receptor such as the amino acid phenylalanine which both lead to an increase in the rate of acid secretion through the apical H+, K+-ATPase in the absence of secretagogues[18,35]. Inactivation of the Ca2+-sensing receptor by reducing extracellular divalent minerals can also down regulate acid secretion even in the presence of potent secretagogues like histamine[18]. From the results of these studies it has now become apparent that the Ca2+-sensing receptor (CaSR) plays an important regulatory role in acid secretion in mammalian gastric glands.
In addition to the animal studies on gastric glands, the CaSR has also been identified in human gastric tissues[11,16,17,36-38]. In the mucous epithelial cells, activation of the receptor results in a rapid rise in intracellular Ca2+ as well as a proliferative response when the cells were placed in culture[11]. In G cells, stimulation of the receptor results in gastrin release[16] accompanied by activation of phospholipase C[16] and an increase in intracellular Ca2+[16,17]. The associated histamine release due to CaSR-mediated secretion of gastrin by G cells could account for rebound acid secretion that occurs following exposure to calcium containing antacids. All of these data are consistent with the scheme that the CaSR in the stomach could play an important role in both acid secretion and in mucosal repair. Activation of the receptor may act to modulate the rates of acid secretion in response to total body calcium homeostasis. Should there be a deficiency in calcium receptor activation would increase acid secretion, or prolong acid secretion, thereby allow maximal ionization of calcium from ingested foodstuffs and produce increased calcium delivery to the intestine. Increased intestinal calcium will activate the CaSR on the apical surface of the cells and result in inhibition of fluid secretion and enhanced absorption of the delivered Ca2+. Over time as serum calcium rises, gastric CaSR would either become internalized, or deactivated, leading to a down regulation in acid secretion. In patients with Zollinger-Ellison syndrome (ZES; characterized by ulcer disease of the upper gastrointestinal tract, increased gastrin secretion, and non-β-cell tumors of the pancreas, i.e., gastrinomas), gastrin secretion and serum levels appear to correlate with the activity of the frequently associated hyperparathyroidism. This result would be consistent with gastrin secretion paralleling high PTH-driven elevations in plasma Ca2+. Significant albeit variable, CaSR expression has been detected in human gastrinomas[37,38], suggesting that the receptor could mediate the effect of extracellular Ca2+ on gastrin secretion. Consistent with this explanation, activation of CaSR by raising extracellular Ca2+ increased rapidly intracellular Ca2+ that was not altered by the Ca2+ channel blocker, nifedipine[38].
The Ca2+-sensing receptor is expressed in epithelial cells along the entire small and large intestine, but only in colon has the receptor been studied in sufficient detail to permit comment on potential roles in normal intestinal function, in diarrheal states, and the effect of oral Ca2+ intake on reducing the risk of colon cancer. The expression of CaSR in nerve plexi involved in smooth muscle function and coordination, however, suggests a potential role in modulating intestinal motility. The latter could be important in coordinating food delivery (Ca2+, amino acids, polyamines) and modulating intestinal motility to maximize nutrient absorption. In addition, an effect of CaSR activation on intestinal motility may be one factor contributing to the constipation that is associated with hypercalcemic states.
The primary function of the colon is to both absorb and secrete fluid and thereby maintain normal salt and water homeostasis. The colon is a complex epithelium that consists of both extensive invaginations of the surface which are designated as crypts which make up approximately 90% of the epithelial mass and the remaining 10% being surface cells. Although earlier studies suggested that only surface cells absorb and only crypt cells secrete fluid into the lumen of the colon, recent evidence has established that both surface and crypt cells absorb and secrete fluid (see review for details;[39]). As over 90% of the colonic epithelial surface area is occupied by invaginations or crypts, these structures constitute the major functional unit of the colonic epithelium[40,41].
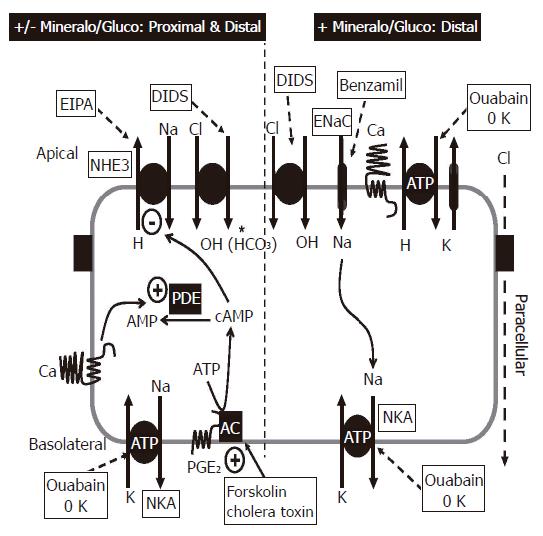
The direction of net fluid transport is determined by the relative magnitudes of the absorptive and secretory fluxes. Under basal conditions (absence of hormones, drugs or other factors), net fluid transport by crypts is absorptive[42]. However, colonic crypts alter the direction of net fluid transport to secretion upon exposure to cell permeable cyclic AMP analogues, (forskolin, or other agents that activate adenylate cyclase), or modulators of cyclic AMP metabolism such as phosphodiesterase (PDE) inhibitors[42]. Addition of cyclic AMP-generating hormones/factors like 5-hydroxytryptophan or prostaglandin E2 to the blood/interstitial surface of the crypt will also increases fluid secretion by colonic crypts[40,41]. Modulation of these fluid transport processes in the colon by cyclic AMP can result in profound fluid and electrolyte losses with associated secretory diarrheas, as is the case during cholera exposure (Figure 3)[40].
Some previous physiological studies in rats, measuring Ca2+ fluxes in isolated colonic mucosa suggested that the colon had the capacity to respond to changes in extracellular Ca2+. For example, the colon, as is true for the small intestine, can absorb and secrete Ca2+ in response to changes in extracellular Ca2+ as well as in levels of 1, 25-dihydroxy vitamin D3[43-45]. These latter observations indicate that colonic mucosal epithelium per se is equipped with a Ca2+-sensing mechanism. Recent studies have suggested that this divalent mineral sensing mechanism in colonic epithelia is the CaSR, based on immunolocalization of the receptor in apical and basolateral membranes and receptor function assays[3,8,9,12].
The activation of colonic CaSR by: Ca2+, Gd3+ or neomycin, leads to rapid rises in intracellular Ca2+ in both surface and crypt cells[5]. The elevation in intracellular Ca2+ occurs within a few seconds, consistent with activation of the phosphatidylinositolphospholipase C-inositol 1, 4, 5-trisphosphate (PI-PLC-IP3) pathway by G protein-coupled cell membrane receptors. Ca2+-sensing receptor-mediated increases in intracellular Ca2+ can be prevented by pre-treatment with U-73122, a specific inhibitor of phosphatidylinositolphospholipase C. This effect of PLC inhibition demonstrated that intracellular Ca2+ transients induced by Ca2+-sensing receptor agonists were not the result of altered entry of extracellular Ca2+ into colonic epithelial cells but were rather due to receptor-mediated activation of PI-PLC. The receptor-mediated increase in intracellular Ca2+ concentration in colon was shown to be due to the release of Ca2+ from thapsigargin-sensitive cell stores[5].
The role of CaSR in modulating colonic fluid movement has been examined in isolated perfused colonic crypts using an in vitro micro-perfusion technique[5,46]. Under basal conditions (i.e., in the absence of forskolin, or other secretagogues), crypts exhibit net fluid absorption[47]. Following exposure to forskolin a net fluid secretion occurs[47]. Activation of either luminal or basolateral CaSR by Ca2+ and/or spermine reverses the forskolin-stimulated fluid secretion[5,46]. Further studies will be necessary to fully define the mechanism of CaSR effects on cyclic AMP-mediated fluid secretion. However, based on information recently obtained from the effects of CaSR activation on vasopressin-stimulated increases in cyclic AMP in the kidney thick ascending limb of Henle[48,49], we postulate CaSR-mediated elevation in intracellular Ca2+ would activate Ca2+/calmodulin-sensitive phosphodiesterases that would metabolize intracellular cyclic AMP, and thereby abrogate fluid secretion (Figure 3).
Increasing the levels of calcium on either the apical or basolateral membrane of the intact colon in Ussing chambers or in isolated perfused crypts leads to a decrease in fluid secretion. This decrease remains even in the presence of potent secretagogues such as forskolin; in fact there is enhanced absorption of fluid in the continued presence of potent secretagogues as long as the receptor remains activated[5,6]. In disease states or infectious states, fluid and electrolyte secretion can occur at pronounced levels and can cause dehydration and potentially death. By modulating the CaSR through increased delivery of calcium, or calcimimetic agents to the receptor, it appears possible that secretion could be stopped. This aspect of the CaSR could serve as an important new therapeutic target to modulate secretion and absorption of electrolytes along the intestine and combat secretory disease states.
The epithelium of the colon and the small intestine remains in a constant state of renewal. In the colon cells proliferate and become differentiated as they migrate from the base of the crypt towards the surface. Therefore cells at the base of the crypt are highly proliferative but less differentiated, whereas cells along the surface of the colon are highly differentiated and are in a non-proliferative state. Alterations of this tightly regulated process may lead to the development of hyperplastic events (polyps) and/or tumors. A potential role for the Ca2+-sensing receptor in colonic epithelial cell proliferation, differentiation and development is suggested by the observations that receptor activation reduces proliferation and induces differentiation of a variety of different cell types in addition to the intestinal epithelium. For example, activation of CaSR enhances cell differentiation in both mouse[50,51] and human[52,53] keratinocytes[54]. Moreover, activation of the Ca2+-sensing receptor in other cells modulates proliferation and inhibits apoptosis[55-57].
The Ca2+-sensing receptor responds not only to changes in divalent minerals but also to changes in organic nutrients (such as polyamines)[58] and amino acids[59,60]. Recently this effect was demonstrated in isolated rat gastric glands, whereby stimulation of the Ca2+-sensing receptor by L-amino acids induced acid secretion in vitro[35]. In addition to activation of CaSR, there is evidence that certain amino acids can stimulate gastric acid secretion via the system L-amino acid transporter [Kirchhoff] which illustrates an increased layer of complexity in the process of acid secretion. Organic nutrients function primarily by altering the EC50 of the CaSR for Ca2+, although direct agonist effects have been demonstrated. These nutrients could potentially alter the conformational structure of the Ca2+-sensing receptor thereby enhancing the affinity for divalent ions and attenuating the cellular effects of receptor stimulation. The potential roles for these nutrients in coordinating protein and divalent mineral metabolism and in providing information on nutrient delivery to intestinal cells will be discussed in the following section.
In small rodents such as rats and mice , dietary polyamine intake plays an essential role for normal gastrointestinal tract cell growth and development[61-65]. In humans, the postulated mechanisms for the pro-differentiation and anti-cancer effects of dietary Ca2+/polyamines include: (1) formation of insoluble salts of Ca2+ with otherwise tumorigenic fatty acids and bile salts; and (2) modulation of the rates and/or fates of biologically active molecules such as nucleic acids, proteins and phospholipids[66-69]. The presence of CaSR on the plasma membranes of both surface and crypt epithelial cells raises the intriguing possibility that this receptor could mediate some of the dietary effects of Ca2+, polyamines, and other nutrients on tissue modeling of intestinal epithelia.
Increases in polyamines, specifically spermine, results in the generation of IP3, raises intracellular Ca2+, and modulates forskolin-stimulated fluid secretion, all consistent with activation of the colonic epithelial CaSR[46]. Polyamine (spermine > spermidine > putrescine)-mediated augmentation of intracellular IP3 and Ca2+ accumulation requires the presence of, and is potentiated by, extracellular Ca2+. The EC50 for Ca2+o- mediated activation of the CaSR was also reduced by polyamines[46]. These results demonstrate that the colonic epithelial CaSR also positively responds to polyamines.
In cultured intestinal cell lines, CaSR has been shown to increase E-cadherin and reduce β-catenin production which are markers for intestinal differentiation[14,70,71]. In Caco-2 cells expressing the CaSR activation of this receptor by extracellular Ca2+ increases thymidine incorporation into DNA as a marker of cell proliferation[72]. Low concentrations of extracellular Ca2+ cause a PKC-dependent increase in c-myc protooncogene expression in Caco-2 cells and this pro-proliferative effect is abrogated by activation of the CaSR by increasing concentrations of extracellular Ca2+[15]. The CaSR in keratinocytes and certain other cells has been shown to alter proliferation/differentiation and to modulate the activities of MAP and tyrosine kinases associated with cell proliferation[50-52,57,72-77]. All of these data, when taken together, support a potential role for the CaSR as a modulator of cell proliferation and differentiation in intestinal epithelial cells.
Ingestion of high dietary Ca2+ promotes colonic mucosal epithelial cell differentiation, decreases cell growth, and reduces the risk for development of colorectal cancer (see recent summaries[78,79] and[14,15,77,80,81] and review by Karen Roland Cell Calcium special issue). Cancer of the colon and rectum is the second most frequently diagnosed malignancy in the United States in addition to being the second most common cause of cancer-related death (>56 000 American deaths this year). Observations that demonstrate that increases in dietary calcium reduce the risk of developing colon adenomas are noteworthy. Specifically, by increasing dietary calcium intake there has been: (1) a reduced risk for colorectal cancer by three-fold in men consuming 1400-1500 mg calcium per day, 19 year prospective study of men working at the Western Electric Co., Chicago[82]; (2) a significant reduction in colonic crypt cell proliferation and enhanced markers of cell differentiation in human subjects at increased risk for colon cancer[81,83,84]; (3) a reduced risk of colorectal adenomas in humans[85] also see[86-89]; (4) decrease in the incidence and number of carcinogen-induced colonic tumors in virtually all studies in rats, see[90] for a review; (5) significantly reduced recurrence of colorectal adenomas in a randomized, double-blind trial of 930 subjects[85]; (6) long term calcium supplements significantly suppressed colonic cell proliferation in adenoma patients[80]. Activation of the CaSR in human carcinoma cell lines by raising extracellular Ca2+ promotes E-cadherin expression while suppressing β-catenin activation[14], both markers of cell differentiation[70,71]. In addition, there has also been a correlation between CaSR expression and the stage of differentiation n human colon tumors[14]. These observations provide a significant body of evidence that increases in dietary Ca2+ reduce the risk of colon cancer and are mediated by activation of the CaSR.
The ability of the CaSR to be activated by l-amino acids has been suggested as a link between protein and calcium metabolism[60]. This was suggested by the direct relationship between dietary protein intake and renal Ca2+ excretion[91,92]. A diet high in protein acutely increased urinary Ca2+ excretion[91,92] and a low protein intake induces elevated parathyroid hormone levels[93]. The increased urinary Ca2+ excretion associated with high protein intake appears due to elevations in intestinal Ca2+ absorption[91-93], although this has not been a universal finding[94]. As discussed in previous sections of this review, activation of the CaSR by Ca2+ stimulates gastric acid secretion, which in turn would promote acid digestion of proteins (together with peptidases). The release of l-amino acids would then promote Ca2+ absorption in small and large intestine by their synergistic activation of the Ca2+-sensing receptor.
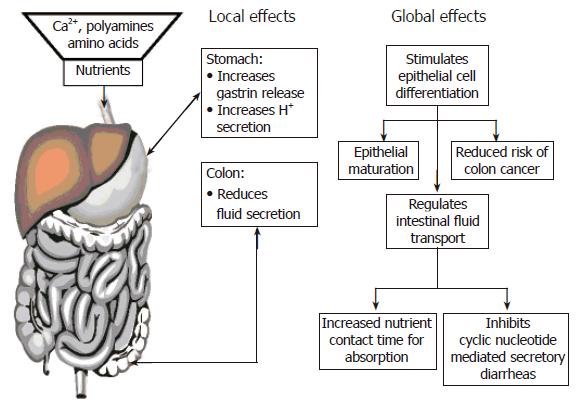
In summary, Figure 4 presents a current summary of the potential roles of the CaSR in gastrointestinal biology. Because of the unique properties of the CaSR in recognizing and responding to extracellular Ca2+o and nutrients, this receptor presents a potential mechanism linking dietary metabolism (i.e., food digestion and nutrient absorption) to: (1) nutrient availability for epithelial growth and differentiation; and (2) protein and divalent mineral metabolism; (3) dietary Ca2+ intake and the associated reduction in risk of colon cancer; and (4) nutrient, salt and fluid homeostasis. In addition, the potential effects of nutrient activation of the CaSR on intestinal motility, coupled to the demonstrated reduction in fluid secretion, would increase nutrient-epithelial contact time and thereby enhance absorption. Finally, the potent ability of CaSR agonists to abrogate cyclic AMP-mediated fluid secretion by the colon has important implications for development of novel oral therapies of cyclic nucleotide dependent diarrheas like cholera.
S- Editor Pan BR E- Editor Liu WF
| 1. | Nearing J, Betka M, Quinn S, Hentschel H, Elger M, Baum M, Bai M, Chattopadyhay N, Brown EM, Hebert SC. Polyvalent cation receptor proteins (CaRs) are salinity sensors in fish. Proc Natl Acad Sci U S A. 2002;99:9231-9236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 104] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Diaz R, Hurwitz S, Chattopadhyay N, Pines M, Yang Y, Kifor O, Einat MS, Butters R, Hebert SC, Brown EM. Cloning, expression, and tissue localization of the calcium-sensing receptor in chicken (Gallus domesticus). Am J Physiol. 1997;273:R1008-R1016. [PubMed] |
| 3. | Cima RR, Cheng I, Klingensmith ME, Chattopadhyay N, Kifor O, Hebert SC, Brown EM, Soybel DI. Identification and functional assay of an extracellular calcium-sensing receptor in Necturus gastric mucosa. Am J Physiol. 1997;273:G1051-G1060. [PubMed] |
| 4. | Caroppo R, Gerbino A, Debellis L, Kifor O, Soybel DI, Brown EM, Hofer AM, Curci S. Asymmetrical, agonist-induced fluctuations in local extracellular [Ca(2+)] in intact polarized epithelia. EMBO J. 2001;20:6316-6326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Cheng SX, Okuda M, Hall AE, Geibel JP, Hebert SC. Expression of calcium-sensing receptor in rat colonic epithelium: evidence for modulation of fluid secretion. Am J Physiol Gastrointest Liver Physiol. 2002;283:G240-G250. [PubMed] |
| 6. | Cheng I, Qureshi I, Chattopadhyay N, Qureshi A, Butters RR, Hall AE, Cima RR, Rogers KV, Hebert SC, Geibel JP. Expression of an extracellular calcium-sensing receptor in rat stomach. Gastroenterology. 1999;116:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Chattopadhyay N, Cheng I, Rogers K, Riccardi D, Hall A, Diaz R, Hebert SC, Soybel DI, Brown EM. Identification and localization of extracellular Ca(2+)-sensing receptor in rat intestine. Am J Physiol. 1998;274:G122-G130. [PubMed] |
| 8. | Gama L, Baxendale-Cox LM, Breitwieser GE. Ca2+-sensing receptors in intestinal epithelium. Am J Physiol. 1997;273:C1168-C1175. [PubMed] |
| 9. | Mitsuma T, Rhue N, Kayama M, Mori Y, Adachi K, Yokoi Y, Ping J, Nogimori T, Hirooka Y. Distribution of calcium sensing receptor in rats: an immunohistochemical study. Endocr Regul. 1999;33:55-59. [PubMed] |
| 10. | Butters RR Jr, Chattopadhyay N, Nielsen P, Smith CP, Mithal A, Kifor O, Bai M, Quinn S, Goldsmith P, Hurwitz S. Cloning and characterization of a calcium-sensing receptor from the hypercalcemic New Zealand white rabbit reveals unaltered responsiveness to extracellular calcium. J Bone Miner Res. 1997;12:568-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Rutten MJ, Bacon KD, Marlink KL, Stoney M, Meichsner CL, Lee FP, Hobson SA, Rodland KD, Sheppard BC, Trunkey DD. Identification of a functional Ca2+-sensing receptor in normal human gastric mucous epithelial cells. Am J Physiol. 1999;277:G662-G670. [PubMed] |
| 12. | Sheinin Y, Kállay E, Wrba F, Kriwanek S, Peterlik M, Cross HS. Immunocytochemical localization of the extracellular calcium-sensing receptor in normal and malignant human large intestinal mucosa. J Histochem Cytochem. 2000;48:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Hentschel H, Nearing J, Harris HW, Betka M, Baum M, Hebert SC, Elger M. Localization of Mg2+-sensing shark kidney calcium receptor SKCaR in kidney of spiny dogfish, Squalus acanthias. Am J Physiol Renal Physiol. 2003;285:F430-F439. [PubMed] |
| 14. | Chakrabarty S, Radjendirane V, Appelman H, Varani J. Extracellular calcium and calcium sensing receptor function in human colon carcinomas: promotion of E-cadherin expression and suppression of beta-catenin/TCF activation. Cancer Res. 2003;63:67-71. [PubMed] |
| 15. | Kállay E, Bajna E, Wrba F, Kriwanek S, Peterlik M, Cross HS. Dietary calcium and growth modulation of human colon cancer cells: role of the extracellular calcium-sensing receptor. Cancer Detect Prev. 2000;24:127-136. [PubMed] |
| 16. | Buchan AM, Squires PE, Ring M, Meloche RM. Mechanism of action of the calcium-sensing receptor in human antral gastrin cells. Gastroenterology. 2001;120:1128-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Ray JM, Squires PE, Curtis SB, Meloche MR, Buchan AM. Expression of the calcium-sensing receptor on human antral gastrin cells in culture. J Clin Invest. 1997;99:2328-2333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 127] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Geibel JP, Wagner CA, Caroppo R, Qureshi I, Gloeckner J, Manuelidis L, Kirchhoff P, Radebold K. The stomach divalent ion-sensing receptor scar is a modulator of gastric acid secretion. J Biol Chem. 2001;276:39549-39552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 61] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Hirschowitz BI, Keeling D, Lewin M, Okabe S, Parsons M, Sewing K, Wallmark B, Sachs G. Pharmacological aspects of acid secretion. Dig Dis Sci. 1995;40:3S-23S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Prinz C, Kajimura M, Scott D, Helander H, Shin J, Besancon M, Bamberg K, Hersey S, Sachs G. Acid secretion and the H,K ATPase of stomach. Yale J Biol Med. 1992;65:577-596. [PubMed] |
| 21. | Hersey SJ, Sachs G. Gastric acid secretion. Physiol Rev. 1995;75:155-189. [PubMed] |
| 22. | Sachs G, Prinz C, Loo D, Bamberg K, Besancon M, Shin JM. Gastric acid secretion: activation and inhibition. Yale J Biol Med. 1994;67:81-95. [PubMed] |
| 23. | Scott DR, Helander HF, Hersey SJ, Sachs G. The site of acid secretion in the mammalian parietal cell. Biochim Biophys Acta. 1993;1146:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Gedda K, Scott D, Besancon M, Lorentzon P, Sachs G. Turnover of the gastric H+,K(+)-adenosine triphosphatase alpha subunit and its effect on inhibition of rat gastric acid secretion. Gastroenterology. 1995;109:1134-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Forte JG, Adams PH, Davies RE. Acid secretion and phosphate metabolism in bullfrog gastric mucosa. Biochim Biophys Acta. 1965;104:25-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Forte JG. Recent concepts of the mechanism of hydrochloric acid secretion. J Indian Med Prof. 1966;12:5637-5647. [PubMed] |
| 27. | Rong Q, Bastaki SM, Forte JG. An improved method to evaluate secretory activity of isolated gastric glands and cells. Dig Dis Sci. 2002;47:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Yamaji R, Sakamoto M, Miyatake K, Nakano Y. Hypoxia inhibits gastric emptying and gastric acid secretion in conscious rats. J Nutr. 1996;126:673-680. [PubMed] |
| 29. | Sachs G, Shin JM, Besancon M, Prinz C. The continuing development of gastric acid pump inhibitors. Aliment Pharmacol Ther. 1993;7 Suppl 1:4-12, discussion 29-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Cuppoletti J, Sachs G. Regulation of gastric acid secretion via modulation of a chloride conductance. J Biol Chem. 1984;259:14952-14959. [PubMed] |
| 31. | Malinowska DH, Cuppoletti J, Sachs G. Cl- requirement of acid secretion in isolated gastric glands. Am J Physiol. 1983;245:G573-G581. [PubMed] |
| 32. | Banerjee S, El-Omar E, Mowat A, Ardill JE, Park RH, Watson W, Beattie AD, McColl KE. Sucralfate suppresses Helicobacter pylori infection and reduces gastric acid secretion by 50% in patients with duodenal ulcer. Gastroenterology. 1996;110:717-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Geibel J, Abraham R, Modlin I, Sachs G. Gastrin-stimulated changes in Ca2+ concentration in parietal cells depends on adenosine 3',5'-cyclic monophosphate levels. Gastroenterology. 1995;109:1060-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Urushidani T, Forte JG. Signal transduction and activation of acid secretion in the parietal cell. J Membr Biol. 1997;159:99-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Busque SM, Kerstetter JE, Geibel JP, Insogna K. L-type amino acids stimulate gastric acid secretion by activation of the calcium-sensing receptor in parietal cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G664-G669. [PubMed] |
| 36. | Dufner MM, Kirchhoff P, Remy C, Hafner P, Müller MK, Cheng SX, Tang LQ, Hebert SC, Geibel JP, Wagner CA. The calcium-sensing receptor acts as a modulator of gastric acid secretion in freshly isolated human gastric glands. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1084-G1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Goebel SU, Peghini PL, Goldsmith PK, Spiegel AM, Gibril F, Raffeld M, Jensen RT, Serrano J. Expression of the calcium-sensing receptor in gastrinomas. J Clin Endocrinol Metab. 2000;85:4131-4137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Itami A, Kato M, Komoto I, Doi R, Hosotani R, Shimada Y, Imamura M. Human gastrinoma cells express calcium-sensing receptor. Life Sci. 2001;70:119-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245-289. [PubMed] |
| 40. | Raufman JP. Cholera. Am J Med. 1998;104:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Sandle GI. Salt and water absorption in the human colon: a modern appraisal. Gut. 1998;43:294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 109] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Binder HJ, Singh SK, Geibel JP, Rajendran VM. Novel transport properties of colonic crypt cells: fluid absorption and Cl-dependent Na-H exchange. Comp Biochem Physiol A Physiol. 1997;118:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Favus MJ, Kathpalia SC, Coe FL. Kinetic characteristics of calcium absorption and secretion by rat colon. Am J Physiol. 1981;240:G350-G354. [PubMed] |
| 44. | Lee DB, Walling MW, Gafter U, Silis V, Coburn JW. Calcium and inorganic phosphate transport in rat colon: dissociated response to 1,25-dihydroxyvitamin D3. J Clin Invest. 1980;65:1326-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Nellans HN, Kimberg DV. Anomalous calcium secretion in rat ileum: role of paracellular pathway. Am J Physiol. 1979;236:E473-E481. [PubMed] |
| 46. | Cheng SX, Geibel JP, Hebert SC. Extracellular polyamines regulate fluid secretion in rat colonic crypts via the extracellular calcium-sensing receptor. Gastroenterology. 2004;126:148-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Singh SK, Binder HJ, Boron WF, Geibel JP. Fluid absorption in isolated perfused colonic crypts. J Clin Invest. 1995;96:2373-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 48. | de Jesus Ferreira MC, Héliès-Toussaint C, Imbert-Teboul M, Bailly C, Verbavatz JM, Bellanger AC, Chabardès D. Co-expression of a Ca2+-inhibitable adenylyl cyclase and of a Ca2+-sensing receptor in the cortical thick ascending limb cell of the rat kidney. Inhibition of hormone-dependent cAMP accumulation by extracellular Ca2+. J Biol Chem. 1998;273:15192-15202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | De Jesus Ferreira MC, Bailly C. Extracellular Ca2+ decreases chloride reabsorption in rat CTAL by inhibiting cAMP pathway. Am J Physiol. 1998;275:F198-F203. [PubMed] |
| 50. | Oda Y, Tu CL, Chang W, Crumrine D, Kömüves L, Mauro T, Elias PM, Bikle DD. The calcium sensing receptor and its alternatively spliced form in murine epidermal differentiation. J Biol Chem. 2000;275:1183-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Oda Y, Tu CL, Pillai S, Bikle DD. The calcium sensing receptor and its alternatively spliced form in keratinocyte differentiation. J Biol Chem. 1998;273:23344-23352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 122] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Tu CL, Oda Y, Bikle DD. Effects of a calcium receptor activator on the cellular response to calcium in human keratinocytes. J Invest Dermatol. 1999;113:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Bikle DD, Ratnam A, Mauro T, Harris J, Pillai S. Changes in calcium responsiveness and handling during keratinocyte differentiation. Potential role of the calcium receptor. J Clin Invest. 1996;97:1085-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 159] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 54. | Bikle DD, Ng D, Tu CL, Oda Y, Xie Z. Calcium- and vitamin D-regulated keratinocyte differentiation. Mol Cell Endocrinol. 2001;177:161-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 128] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 55. | Mailland M, Waelchli R, Ruat M, Boddeke HG, Seuwen K. Stimulation of cell proliferation by calcium and a calcimimetic compound. Endocrinology. 1997;138:3601-3605. [PubMed] [DOI] [Full Text] |
| 56. | Lin KI, Chattopadhyay N, Bai M, Alvarez R, Dang CV, Baraban JM, Brown EM, Ratan RR. Elevated extracellular calcium can prevent apoptosis via the calcium-sensing receptor. Biochem Biophys Res Commun. 1998;249:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | McNeil SE, Hobson SA, Nipper V, Rodland KD. Functional calcium-sensing receptors in rat fibroblasts are required for activation of SRC kinase and mitogen-activated protein kinase in response to extracellular calcium. J Biol Chem. 1998;273:1114-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 162] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 58. | Quinn SJ, Ye CP, Diaz R, Kifor O, Bai M, Vassilev P, Brown E. The Ca2+-sensing receptor: a target for polyamines. Am J Physiol. 1997;273:C1315-C1323. [PubMed] |
| 59. | Conigrave AD, Quinn SJ, Brown EM. L-amino acid sensing by the extracellular Ca2+-sensing receptor. Proc Natl Acad Sci U S A. 2000;97:4814-4819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 369] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 60. | Conigrave AD, Franks AH, Brown EM, Quinn SJ. L-amino acid sensing by the calcium-sensing receptor: a general mechanism for coupling protein and calcium metabolism. Eur J Clin Nutr. 2002;56:1072-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Buts JP, De Keyser N, Kolanowski J, Sokal E, Van Hoof F. Maturation of villus and crypt cell functions in rat small intestine. Role of dietary polyamines. Dig Dis Sci. 1993;38:1091-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Löser C, Eisel A, Harms D, Fölsch UR. Dietary polyamines are essential luminal growth factors for small intestinal and colonic mucosal growth and development. Gut. 1999;44:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 63. | Dufour C, Dandrifosse G, Forget P, Vermesse F, Romain N, Lepoint P. Spermine and spermidine induce intestinal maturation in the rat. Gastroenterology. 1988;95:112-116. [PubMed] |
| 64. | Bardócz S, Duguid TJ, Brown DS, Grant G, Pusztai A, White A, Ralph A. The importance of dietary polyamines in cell regeneration and growth. Br J Nutr. 1995;73:819-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 182] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 65. | Osborne DL, Seidel ER. Microflora-derived polyamines modulate obstruction-induced colonic mucosal hypertrophy. Am J Physiol. 1989;256:G1049-G1057. [PubMed] |
| 66. | Seiler N, Delcros JG, Moulinoux JP. Polyamine transport in mammalian cells. An update. Int J Biochem Cell Biol. 1996;28:843-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 286] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 67. | Pegg AE. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988;48:759-774. [PubMed] |
| 68. | Marton LJ, Pegg AE. Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol Toxicol. 1995;35:55-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 510] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 69. | Pegg AE, McCann PP. Polyamine metabolism and function. Am J Physiol. 1982;243:C212-C221. [PubMed] |
| 70. | Van Aken E, De Wever O, Correia da Rocha AS, Mareel M. Defective E-cadherin/catenin complexes in human cancer. Virchows Arch. 2001;439:725-751. [PubMed] |
| 71. | Wong NA, Pignatelli M. Beta-catenin--a linchpin in colorectal carcinogenesis. Am J Pathol. 2002;160:389-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 265] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 72. | Kállay E, Kifor O, Chattopadhyay N, Brown EM, Bischof MG, Peterlik M, Cross HS. Calcium-dependent c-myc proto-oncogene expression and proliferation of Caco-2 cells: a role for a luminal extracellular calcium-sensing receptor. Biochem Biophys Res Commun. 1997;232:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 73. | Yamaguchi T, Chattopadhyay N, Kifor O, Sanders JL, Brown EM. Activation of p42/44 and p38 mitogen-activated protein kinases by extracellular calcium-sensing receptor agonists induces mitogenic responses in the mouse osteoblastic MC3T3-E1 cell line. Biochem Biophys Res Commun. 2000;279:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 74. | Yamaguchi T, Chattopadhyay N, Kifor O, Butters RR Jr, Sugimoto T, Brown EM. Mouse osteoblastic cell line (MC3T3-E1) expresses extracellular calcium (Ca2+o)-sensing receptor and its agonists stimulate chemotaxis and proliferation of MC3T3-E1 cells. J Bone Miner Res. 1998;13:1530-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 194] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 75. | Yamaguchi T, Yamauchi M, Sugimoto T, Chauhan D, Anderson KC, Brown EM, Chihara K. The extracellular calcium Ca2+o-sensing receptor is expressed in myeloma cells and modulates cell proliferation. Biochem Biophys Res Commun. 2002;299:532-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 76. | Hobson SA, McNeil SE, Lee F, Rodland KD. Signal transduction mechanisms linking increased extracellular calcium to proliferation in ovarian surface epithelial cells. Exp Cell Res. 2000;258:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | Kifor O, MacLeod RJ, Diaz R, Bai M, Yamaguchi T, Yao T, Kifor I, Brown EM. Regulation of MAP kinase by calcium-sensing receptor in bovine parathyroid and CaR-transfected HEK293 cells. Am J Physiol Renal Physiol. 2001;280:F291-F302. [PubMed] |
| 78. | Bresalier RS. Calcium, chemoprevention, and cancer: a small step forward (a long way to go). Gastroenterology. 1999;116:1261-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 79. | Lamprecht SA, Lipkin M. Cellular mechanisms of calcium and vitamin D in the inhibition of colorectal carcinogenesis. Ann N Y Acad Sci. 2001;952:73-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 148] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 80. | Rozen P, Lubin F, Papo N, Knaani J, Farbstein H, Farbstein M, Zajicek G. Calcium supplements interact significantly with long-term diet while suppressing rectal epithelial proliferation of adenoma patients. Cancer. 2001;91:833-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 81. | Holt PR, Atillasoy EO, Gilman J, Guss J, Moss SF, Newmark H, Fan K, Yang K, Lipkin M. Modulation of abnormal colonic epithelial cell proliferation and differentiation by low-fat dairy foods: a randomized controlled trial. JAMA. 1998;280:1074-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 101] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 82. | Garland C, Shekelle RB, Barrett-Connor E, Criqui MH, Rossof AH, Paul O. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet. 1985;1:307-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 405] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 83. | Holt PR, Lipkin M, Newmark H. Calcium intake and colon cancer biomarkers. JAMA. 1999;281:1172-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 84. | Ahnen DJ, Byers T. Proliferation happens. JAMA. 1998;280:1095-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 85. | Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, Rothstein R, Summers RW, Snover DC, Beck GJ. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999;340:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 537] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 86. | Lipkin M, Newmark H. Calcium and the prevention of colon cancer. J Cell Biochem Suppl. 1995;22:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 54] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 87. | Slattery ML, Sorenson AW, Ford MH. Dietary calcium intake as a mitigating factor in colon cancer. Am J Epidemiol. 1988;128:504-514. [PubMed] |
| 88. | Duris I, Hruby D, Pekarkova B, Huorka M, Cernakova E, Bezayova T, Ondrejka P. Calcium chemoprevention in colorectal cancer. Hepatogastroenterology. 1996;43:152-154. [PubMed] |
| 89. | Pence BC. Role of calcium in colon cancer prevention: experimental and clinical studies. Mutat Res. 1993;290:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 90. | Lipkin M, Newmark H. Development of clinical chemoprevention trials. J Natl Cancer Inst. 1995;87:1275-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 91. | Kerstetter JE, O'Brien KO, Insogna KL. Dietary protein, calcium metabolism, and skeletal homeostasis revisited. Am J Clin Nutr. 2003;78:584S-592S. [PubMed] |
| 92. | Kerstetter JE, O'Brien KO, Insogna KL. Dietary protein affects intestinal calcium absorption. Am J Clin Nutr. 1998;68:859-865. [PubMed] |
| 93. | Kerstetter JE, Svastisalee CM, Caseria DM, Mitnick ME, Insogna KL. A threshold for low-protein-diet-induced elevations in parathyroid hormone. Am J Clin Nutr. 2000;72:168-173. [PubMed] |
| 94. | Heaney RP. Dietary protein and phosphorus do not affect calcium absorption. Am J Clin Nutr. 2000;72:758-761. [PubMed] |