Published online Jan 14, 2006. doi: 10.3748/wjg.v12.i2.313
Revised: June 28, 2005
Accepted: July 8, 2005
Published online: January 14, 2006
AIM: To analyze the results and complications of intra-operative enteroscopy (IOE) by investigating a series of selected patients with bleeding suspected to originate from the small intestine.
METHODS: Eighty-one patients (mean age: 65 years) including 40 males (49.4%) and 41 females (50.6%) with obscure gastrointestinal bleeding underwent IOE between 1990 and 2004. The patients were identified from a database and data were selected from the patients’ charts retrospectively. All the patients had undergone at least one non-diagnostic esophagogastroduodenoscopy, colonoscopy, standard enteroscopy and a negative abdominal ultrasound scan before IOE.
RESULTS: The median minimal hemoglobin level in the patients was 59 + 15 g/L and 72.8% of the patients required transfusion of packed erythrocytes previously. A bleeding source was detected in 68 (84%) of the patients during IOE. Angiodysplasiae were found in 44 patients (54.3%) and 9 patients (11.1%) were affected by ulcers in the small intestine. A tumor in the small intestine was detected in another 6 patients. The treatment consisted of argon-plasma-coagulation, surgical suture or limited resection in most of the patients.
CONCLUSION: Intra-operative enteroscopy is still used for the diagnosis of suspected small bowel bleeding. Recent developments such as wireless capsule endoscopy and double balloon enteroscopy, may lead to the replacement of IOE in the future.
- Citation: Jakobs R, Hartmann D, Benz C, Schilling D, Weickert U, Eickhoff A, Schoenleben K, Riemann JF. Diagnosis of obscure gastrointestinal bleeding by intra-operative enteroscopy in 81 consecutive patients. World J Gastroenterol 2006; 12(2): 313-316
- URL: https://www.wjgnet.com/1007-9327/full/v12/i2/313.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i2.313
In most patients suffering from gastrointestinal bleeding, a bleeding source can be detected using upper endoscopy or colonoscopy. However, the small intestine is a source of bleeding in up to 5% of patients[1,2]. The diagnostic and therapeutic approach to patients with bleeding originating from the small intestine still remains a challenge to all physicians involved in their care.
Several endoscopic and non-endoscopic techniques have been developed for detection of the bleeding sites in the small intestine and their use depends on the severity and time parameters of hemorrhage[3]. In the last few years, wireless capsule endoscopy[4,5] and double-balloon enteroscopy[6,7] have closed the diagnostic gap between colonoscopy and push-enteroscopy for many of the patients. However, intra-operative enteroscopy is still a diagnostic approach that offers the complete endoscopic work-up of the entire small intestine as well as the detection of extramural lesions and the definitive endoscopic or surgical therapy during the same session[8-11].
The aim of our study was to analyze the results of intra-operative enteroscopy for the diagnosis of gastrointestinal bleeding of obscure origin by investigating a large consecutive series of patients in a third referral center.
Between 1990 and 2004, 81 consecutive patients with bleeding of obscure origin underwent intra-operative enteroscopy in our department. Patients undergone IOE were identified from a database of the Department of Gastroenterology. The patient charts were analyzed retrospectively with respect to the basic data, such as previously performed examinations, need for transfusion, type of bleeding (overt or obscure), endoscopic findings through IOE, therapeutic procedures performed and short-term outcome after IOE until discharge from the hospital. Basic patient data are given in Table 1.
| Patients (n) | 81 |
| Age (Yrs) | 65 ± 20,8 |
| Female (n) | 41 (50.6%) |
| Transfusion need (%) | 72.8 |
| Minimal hemoglobin level | 5.9± 1.5 g/dL |
All the patients had at least one episode of gastrointestinal tract bleeding with need for transfusion (obscure or overt bleeding), recurrent minor bleeding episodes without transfusion requirement or chronic iron-deficiency-anemia with suspected bleeding of obscure origin. All the patients were subjected to an endoscopic work-up of the colon and the upper gastrointestinal tract before IOE. They all underwent standard enteroscopy (using a sonde-type or push-type enteroscope (Olympus SIF-100 or XSIF-140Q)) and an abdominal ultrasound scan to exclude malformations of the larger abdominal vessels and/or larger tumors in the abdomen. In a large number of patients, additional methods such as barium enema, MR-enteroclysm and angiography or scintigraphy, were applied prior to IOE.
Intra-operative enteroscopy was routinely carried out under general anesthesia. After a median laparotomy, the small intestine was opened through an incision in the middle third. A surgeon placed the endoscope (Olympus PCF-20; PCF-160 HI or CF-Q140) through a sterile sleeve in the intestine. The endoscope was then pushed up to the duodenum and down into the cecum. If a bleeding source was detected, the lesion was treated endoscopically (e.g. argon-plasma-coagulation) or surgically (e.g. resection) during the same session.
Data were entered with a standard program (Microsoft Excel) and analyzed by the statistical module. Data were expressed as median and range if not stated elsewhere. The study was conducted in accordance with the Declaration of Helsinki.
The median minimal hemoglobin level before IOE was 59+15 g/L and 72.8% of the patients received at least two units (0-50) of packed erythrocytes.
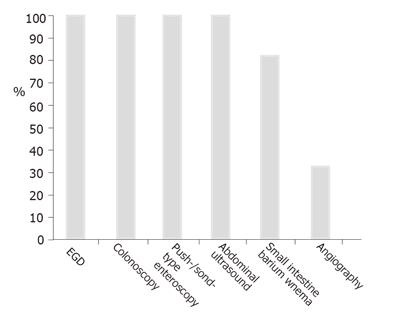
The patients had a median of 4 EGDs and underwent colonoscopies before the IOE procedure. All the patients were subjected to at least one push-type enteroscopy and/or abdominal ultrasound, 80% of them had a non-diagnostic small bowel enema (Figure 1). During the push-enteroscopy prior to IOE, single small angiodysplasiae were found in 5 cases within reach of the enteroscope and treated with argon plasma coagulation. IOE found additional angiodysplasiae. One additional patient had a non-bleeding small ulcer in the upper jejunum and was examined intraoperatively because of ongoing hemorrhage.
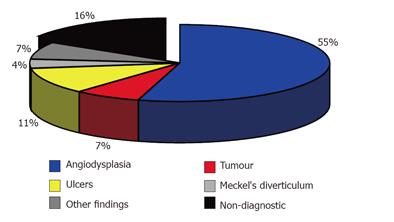
Intra-operative enteroscopy was able to visualize the complete small intestine in all the patients. A bleeding source was detected in 68 (84%) of the patients. Angiodysplasia was found in 44 (54.3%) patients and ulcer in 9 patients (11.1%). The ulcer might be due to the use of non-steroidal anti-rheumatics (NSAR) and the presence of Crohn’s disease. Using IOE, we found tumors of the small intestine not identified previously in 6 patients (7.4%) of the study group. Four of the tumors were malignant, namely 1 B-cell lymphoma, 2 neuroendocrine tumors, 1 gastrointestinal stromal tumor. Two additional tumors were benign, namely 1 Peutz-Jeghers polyp and 1 giant hyperplastic polyp. Four patients of the series had a Meckel’s diverticulum, two of them having a Meckel’s scintigraphy prior IOE without any signs of a Meckel’s diverticulum. Three patients had large diverticula of the jejunum or ileum, which were presumed to be the site of bleeding. Additional diagnoses included Dieulafoy’s ulcer in the cecum (missed by the previously performed colonoscopy) and bleeding from anastomotic vessels after cholecystojejunostomy. Intra-operative enteroscopy was non-diagnostic in 13 patients (16%) (Figure 2).
All patients with a positive finding during IOE were treated. Angiodysplasiae were treated with argon-plasma-coagulation (APC) (n = 20), with a combination of APC and surgical suture in case of large malformations (n = 18), or with surgical resection of a small part of the small intestine (n = 6) in case of multiple angiodysplasiae located in well-defined areas of the ileum. Both the tumors and all the large diverticula were completely resected in all the 6 patients during the IOE session.
One of the patients, an 84-year-old woman with recurrent blood loss originating from the small intestine and a minimal hemoglobin level of 43 g/L, had an intra-abdominal abscess due to a leakage at the incision site of the small intestine. She was re-operated two days after the IOE and recovered soon without further problems. There was no mortality due to the intra-operative enteroscopy or its sequelae during her hospital stay (Figure 3).
The management of patients with obscure gastrointestinal bleeding remains a diagnostic and therapeutic challenge to all physicians involved in their care[3]. After non-diagnostic upper and lower endoscopy, the use of peroral push-type enteroscopy has been the diagnostic standard procedure for many years[12,13].
Push enteroscopy is still the method of choice for suspected small intestinal bleeding as it is easy to perform and is available in several endoscopical centers. Push-enteroscopy provides the option for a broad spectrum of diagnostic and therapeutic endoscopic procedures, including biopsies, argon-plasma-coagulation, injection methods and even removal of polyps[13]. However, this technique is limited with respect to the insertion depth due to the tortuous nature of the small intestine and looping in the stomach. In most cases, only the upper and middle part of the jejunum can be reached and the diagnostic harvest is thus limited. In several series for which push-enteroscopy was used for the diagnosis of a bleeding source, the rate of the diagnostic findings ranges 15-53%, depending on the timing of endoscopy, the type of enteroscope and the clinical setting[5,13-15].
For many years, IOE has been the only method for evaluating the mucosal surface of the complete small intestine. This procedure is limited to patients who are operative candidates and have recurrent or severe bleeding episodes[16], since IOE is an invasive procedure requiring general anesthesia. Several methods for IOE such as peroral approach, transanal and direct intubation through a surgical incision of the small intestine are now available. The latter is method used in our series and allowed for the examination of the entire small intestine in all the patients. In general, the diagnostic yield of IOE is high when patients are highly selected for such procedures. It was reported that IOE can detect 70-80% sources of bleeding[17]. In our series, a probable or definite bleeding source was found in 68 (84%) of the 81 patients. The IOE procedure allowed for the endoscopic or surgical treatment of the lesions during the same session in all our patients. The most common finding by IOE was angiodysplasia with a rate of 54.3%. In several other series investigated for small intestinal bleeding, angiodysplasiae are the most common findings too and local treatment results in the reduction of blood loss in a substantial portion of patients[15,18,19].
Four of the 81 patients had previously non-diagnosed malignant tumors of the small intestine and 6 additional patients were affected by a Meckel’s diverticulum or a large small intestinal diverticulum as a bleeding source. These conditions required operative treatment rather than endoscopic treatment even if they were within the reach of the scope. In our series, we encountered only one major complication during hospital stay (intra-abdominal abscess formation). Most published series present a comparable low morbidity rate and no mortality[8-11,20]. Though the accuracy and diagnostic yield of IOE are high the procedure should be regarded as the last resort for patients with unexplained obscure gastrointestinal bleeding due to its invasive nature[21].
Wireless capsule endoscopy and double-balloon enteroscopy constitute new methods for the diagnosis of patients with obscure gastrointestinal bleeding. Capsule endoscopy allows for non-invasive visualization of the entire small intestine in a substantial portion of patients. It was reported that it has a high diagnostic yield of angiodysplasia, ulcer and active bleeding in selected populations of obscure gastrointestinal bleeding[5]. A recently published Italian multi-center study on capsule endosocpy examined 100 patients with obscure gastrointestinal bleeding and yielded 47% positive and 15% suspicious findings[22]. The timing of capsule endoscopy is important: in patients with ongoing overt bleeding during the examination, the findings are positive in 92%. When capsule endoscopy was performed more than 10 d after an overt bleeding episode, the diagnostic rate decreased to 12%. However, in 21% of cases, the cecum was not reached, and in 5% of the cases, asymptomatic retention of the capsule occurred. Capsule endoscopy is limited in its use because biopsies and interventional treatment procedures are not possible and the localization of findings is sometimes difficult. A two-center-study showed that the sensitivity of capsule endoscopy (compared by IOE as a criterion) is 95%, the positive and negative predictive values are 95% and 86%, respectively[23].
Double balloon enteroscopy (DBE) is a new diagnostic option that has the potential to replace intra-operative enteroscopy in the near future [7]. It allows for endoscopic visualization of the entire small intestine in a substantial portion of patients. The technical principle of DBE is an alternating push and pull procedure[6].
Initial data from Japan and Germany show encouraging results, with a high diagnostic yield of DBE in patients with suspected small bowel diseases. However, in only 10-15% patients, visualization of the complete small intestine is possible. Two procedures, beginning perorally and then transanally, are required for a substantial portion of patients. The procedure is time-consuming. In patients having undergone previous surgical operations of the abdomen, a complete examination of the small intestine is not be possible because of adhesions. As this method is carried out without general anesthesia and its nature is less invasive compared to IOE, repeated DBE is possible even in older patients and in those with recurrent bleeding.
Studies comparing IOE with DBE have not yet been conducted. Such studies may never be performed because patients with a diagnostic DBE do not need an IOE and vice versa. Nevertheless, further studies should be carried out to redefine the role of the invasive IOE procedure in the new era of wireless capsule endoscopy and double-balloon enteroscopy.
This paper was presented in part at the Annual Meeting of the American Society for Gastrointestinal Endoscopy (ASGE) at the Digestive Disease Week 2004 in Orlando/Florida USA (Gastrointest Endosc 2004; 59: AB155)
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Cao L
| 1. | Lewis MP, Khoo DE, Spencer J. Value of laparotomy in the diagnosis of obscure gastrointestinal haemorrhage. Gut. 1995;37:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Ress AM, Benacci JC, Sarr MG. Efficacy of intraoperative enteroscopy in diagnosis and prevention of recurrent, occult gastrointestinal bleeding. Am J Surg. 1992;163:94-8; discussion 98-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 81] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Kovacs TO. Small Bowel Bleeding. Curr Treat Options Gastroenterol. 2005;8:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Mata A, Bordas JM, Feu F, Ginés A, Pellisé M, Fernández-Esparrach G, Balaguer F, Piqué JM, Llach J. Wireless capsule endoscopy in patients with obscure gastrointestinal bleeding: a comparative study with push enteroscopy. Aliment Pharmacol Ther. 2004;20:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Hartmann D, Schilling D, Bolz G, Hahne M, Jakobs R, Siegel E, Weickert U, Adamek HE, Riemann JF. Capsule endoscopy versus push enteroscopy in patients with occult gastrointestinal bleeding. Z Gastroenterol. 2003;41:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | May A, Nachbar L, Wardak A, Yamamoto H, Ell C. Double-balloon enteroscopy: preliminary experience in patients with obscure gastrointestinal bleeding or chronic abdominal pain. Endoscopy. 2003;35:985-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 163] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 7. | Yamamoto H, Kita H, Sunada K, Hayashi Y, Sato H, Yano T, Iwamoto M, Sekine Y, Miyata T, Kuno A. Clinical outcomes of double-balloon endoscopy for the diagnosis and treatment of small-intestinal diseases. Clin Gastroenterol Hepatol. 2004;2:1010-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 427] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 8. | Lau WY. Intraoperative enteroscopy--indications and limitations. Gastrointest Endosc. 1990;36:268-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Whelan RL, Buls JG, Goldberg SM, Rothenberger DA. Intra-operative endoscopy. University of Minnesota experience. Am Surg. 1989;55:281-286. [PubMed] |
| 10. | O'Connel H, Martin CJ. Intra-operative enteroscopy in the management of bleeding small bowel lesions. Aust N Z J Surg. 1992;62:394-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Kendrick ML, Buttar NS, Anderson MA, Lutzke LS, Peia D, Wang KK, Sarr MG. Contribution of intraoperative enteroscopy in the management of obscure gastrointestinal bleeding. J Gastrointest Surg. 2001;5:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Benz C, Jakobs R, Riemann JF. Does the insertion depth in push enteroscopy depend on the working length of the enteroscope. Endoscopy. 2002;34:543-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Wilmer A, Rutgeerts P. Push enteroscopy. Technique, depth, and yield of insertion. Gastrointest Endosc Clin N Am. 1996;6:759-776. [PubMed] |
| 14. | Mylonaki M, Fritscher-Ravens A, Swain P. Wireless capsule endoscopy: a comparison with push enteroscopy in patients with gastroscopy and colonoscopy negative gastrointestinal bleeding. Gut. 2003;52:1122-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 316] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 15. | Van Gossum A, Hittelet A, Schmit A, Francois E, Devière J. A prospective comparative study of push and wireless-capsule enteroscopy in patients with obscure digestive bleeding. Acta Gastroenterol Belg. 2003;66:199-205. [PubMed] |
| 16. | Dulai GS, Jensen DM. Severe gastrointestinal bleeding of obscure origin. Gastrointest Endosc Clin N Am. 2004;14:101-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Douard R, Wind P, Panis Y, Marteau P, Bouhnik Y, Cellier C, Cugnenc P, Valleur P. Intraoperative enteroscopy for diagnosis and management of unexplained gastrointestinal bleeding. Am J Surg. 2000;180:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Adler DG, Knipschield M, Gostout C. A prospective comparison of capsule endoscopy and push enteroscopy in patients with GI bleeding of obscure origin. Gastrointest Endosc. 2004;59:492-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Askin MP, Lewis BS. Push enteroscopic cauterization: long-term follow-up of 83 patients with bleeding small intestinal angiodysplasia. Gastrointest Endosc. 1996;43:580-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Lopez MJ, Cooley JS, Petros JG, Sullivan JG, Cave DR. Complete intraoperative small-bowel endoscopy in the evaluation of occult gastrointestinal bleeding using the sonde enteroscope. Arch Surg. 1996;131:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Cave DR, Cooley JS. Intraoperative enteroscopy. Indications and techniques. Gastrointest Endosc Clin N Am. 1996;6:793-802. [PubMed] |
| 22. | Pennazio M, Santucci R, Rondonotti E, Abbiati C, Beccari G, Rossini FP, De Franchis R. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology. 2004;126:643-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 604] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 23. | Hartmann D, Schmidt H, Bolz G, Schilling D, Kinzel F, Eickhoff A, Huschner W, Möller K, Jakobs R, Reitzig P. A prospective two-center study comparing wireless capsule endoscopy with intraoperative enteroscopy in patients with obscure GI bleeding. Gastrointest Endosc. 2005;61:826-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 201] [Article Influence: 10.1] [Reference Citation Analysis (0)] |