Published online Jan 14, 2006. doi: 10.3748/wjg.v12.i2.234
Revised: June 28, 2005
Accepted: July 15, 2005
Published online: January 14, 2006
AIM: To analyze the localization of erythropoietin receptor on gastric specimens and characterize the effects of erythropoietin on the normal gastric epithelial proliferation using a porcine gastric epithelial cell culture model.
METHODS: Erythropoietin receptor was detected by RT-PCR, Western blotting and immunohistochermistry. Growth stimulation effects of erythropoietin on cultured gastric mucosal cells were determined by ELISA using bromodeoxyuridine (BrdU).
RESULTS: Erythropoietin receptor was detected on cultured porcine gastric mucosal epithelial cells. Erythropoietin receptor was also detected histochemically at the base of gastric mucosal epithelium. BrdU assay demonstrated a dose-dependent increase in growth potential of cultured porcine gastric mucosal epithelial cells by administration of erythropoietin, as well as these effects were inhibited by administration of anti- erythropoietin antibody (P < 0.01).
CONCLUSION: These findings indicate that erythropoietin has a potential to proliferate gastric mucosal epithelium via erythropoietin receptor.
- Citation: Itoh K, Sawasaki Y, Takeuchi K, Kato S, Imai N, Kato Y, Shibata N, Kobayashi M, Moriguchi Y, Higuchi M, Ishihata F, Sudoh Y, Miura S. Erythropoietin -induced proliferation of gastric mucosal cells. World J Gastroenterol 2006; 12(2): 234-239
- URL: https://www.wjgnet.com/1007-9327/full/v12/i2/234.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i2.234
Erythropoietin (EPO) is a glycoprotein (molecular weight = 34 ku) produced in adult kidney and the fetal liver that regulates growth, differentiation, and survival of erythroid progenitors[1]. The effects of EPO are transduced via EPO receptors (EPO-R), members of the cytokine receptor superfamily, that are primarily expressed on the cell surface of erythroid cells[2].
Prior to 1990, morbidity and mortality due to hemorrhagic lesions in dialysis patients was relatively high[3,4]. However, the impact of this complications has been attenuated by the advent of EPO which is used for treatment of renal anemia and by the marked reduction in the incidence peptic ulcer disease due to the eradication of H pylori. Studies on gastric mucosal defense factors have focused on the protective role of gastric mucosal blood flow, oxygen supply, and radical oxygen suppression, and the importance of ferrous ions in the gastric mucosa has also been demonstrated[5,6]. However, direct effects of EPO on the gastric mucosa have not fully been investigated.
Recently, the effect of recombinant human erythropoietin (rhEPO) on cellular proliferation has been examined in human umbilical vein endothelial cells (HUVEC)[7] and bovine pulmonary artery endothelial cells[8]. The growth of these cells was stimulated by EPO. Further, we have previously demonstrated the growth promoting effects of EPO on porcine gastric mucosal vascular endothelial cell[9]. These effects may improve the gastric lesions found in dialysis patients through the neovascularization in mucosal tissue.
Okada et al [10] reported that EPO stimulated proliferation of rat gastric-derived cell lines via EPO-R expressed on these cells. Although these rat stomach derived cells were morphologically epithelioid, they were also immortalized with very short doubling times, which suggests that these cells had undergone transformation and possess characteristics that differ from normal gastric epithelium. Indeed, many types of tumor cells express EPO-R and show increased growth in response to EPO exposure in vitro[11]. Therefore, the goal of the present study was to analyze the localization of EPO-R on gastric epithelium and to characterize the effects of EPO on the normal gastric epithelium using a porcine gastric epithelial cell culture model.
Collagenase and EDTA were purchased from Wako Pure Chemicals (Tokyo, Japan). Keratinocyte-SFM medium, modified Eagle medium (MEM), Dulbecco’s modified minimum essential medium, trypsin, newborn calf serum, TRIzol reagent and penicillin-streptomycin mixture were purchased from Gibco BRL (Gland Island, NY). Human recombinant basic fibroblast growth factor was purchased from Intergen (Purchase, NY). rhEPO (Epoetin Beta) was purchased from Chugai Pharmaceutical Co.(Tokyo, Japan). Monoclonal anti- porcine H+/K+ ATPase antibody was purchased from Affinity Bioreagents (Golden, CO). Anti-porcine stomach pepsin goat antibody was purchased from Polyscinences (Warrington, PA). Monoclonal anti-human gastric mucin antibody (MUC5AC) was purchased from NeoMarkers (Union City, CA). Rabbit anti-human EPO-R antibody was kindly provided by collaborative researcher: Dr. M. Higuchi of Chugai Pharmaceutical Co. (Tokyo, Japan). Isotype matched rabbit IgG against anti-EPO antibody was from Chemicon (Temecula, CA). Horseradish peroxidase-labeled rabbit IgG antibody was purchased from Zymed Laboratories Inc. (South San Francisco, CA). Texas Red labeled anti-mouse IgG and FITC- labeled anti-goat IgG were purchased from Vector Laboratories (Burlingam, CA).
Porcine gastric mucosal epithelial cells were separated by the method with modification which has been developed for the cultivation of human pulmonary epithelial cells[12]. Fetal porcine gastric mucosa was digested with 0.1% collagenase, and cells obtained were seeded onto plastic dishes in plain medium (Dulbecco’s minimum essential medium supplemented with 10% newborn calf serum, 100 U/mL of penicillin and 100 μg/mL of streptomycin) and incubated at 37 °C. The viability of the separated cells was about 80% - 90%. After the epithelial cell colonies grew large enough, other cells were detached from the dish manually with a small piece of silicon rubber under the phase contrast microscopy. The colonies were collected with small grass cylinder (internal diameter 10 mm) and 0.05% trypsin-0.02% EDTA, and the cells were seeded onto new dishes using the growth medium (keratinocyte-SFM containing 10% new born calf serum and 10 ng/mL of basic fibroblast growth factor). The culture medium was changed twice a week. The cells were passaged in the split at the ratio 1:4 and were used in younger generation (passages 6-7).
Cells were also identified as gastric epithelial cells by double staining with anti- porcine H +/K + ATPase antibody, anti-pepsin antibody and anti-gastric mucin antibody in order to identify for gastric parietal cells, chief cells and mucin-producing cells, respectively. Cell growth assessed by the overnight bromodeoxyuridine (BrdU) uptake showed that about 60% of the cells retained growth potential and the population doubling time was about 36 h in these culture conditions.
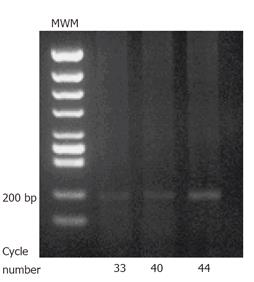
Analysis of EPO-R by RT-PCR: EPO-R gene expression in porcine gastric mucosal epithelial cells (1×107) was analyzed by RT-PCR. Total RNA was extracted using TRIzol reagent. RNA samples were treated with RNase-free DNaseIat 37 °Cfor 30 min to eliminate genomic DNA contamination. RNA was purified with 1 volume of phenol:chloroform (5:1). RT-PCR was performed by RNA PCR Kit (AMV) ver. 2.1 (Takara, Japan). Briefly, 1 μg of RNA was primed with random primers and reverse transcribed using reverse transcriptase in a final volume 20 μL. 5 μL of this mixture was PCR- amplified in a 25 μL reaction using Takara Taq DNA polymerase. The following PCR priming sets were used under PCR conditions of 94 °C for 45 s, 58 °C for 1 min, and 72 °C for 2 min. EPO-R: forward primer (5'-AGC TGT GGC TGT ACC AAA CTG A-3'), reverse primer (5'-ACT TGT CCA GCA CCA GGT AGG T-3'); number of cycles: 36, 40, and 44. All PCR products were separated on ethidium bromide-stained gel.
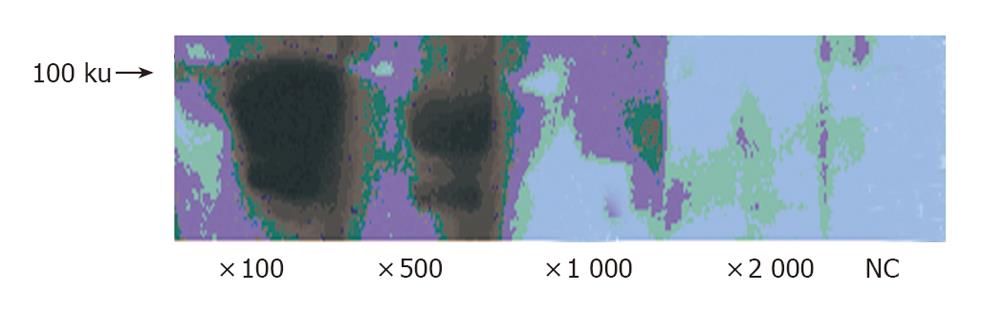
Porcine epithelial cells (0.5 - 1×107) were cultured in modified Eagle’s medium (MEM) containing 10% fetal calf serum (FCS) for 48 h. EPO (1 µg/mL) was added, and the cells were incubated for an additional 24 h. The cells were homogenized in cold buffer. The protein concentration was measured by DC protein assay kit (Bio-Rad, CA) according to the manufacturer’s instruction. Aliquots of 50 µg protein were applied each lane of 10% SDS-polyacrylamide gel and electrophoresed. After electrophoresis, samples were transferred onto PVDF membrane (Millipore, MA). The filter was then incubated with anti-human EPO-R monoclonal antibody (diluted 1:100, 1:500, 1:1 000 and 1:2 000 respectively). Reaction product was demonstrated using ECL-plus Kit (Amersham. Bioscience, UK). For a negative control experiment, the primary antibody was omitted from the solution.
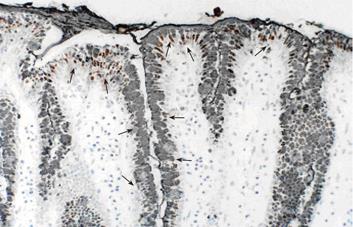
Stomaches were resected from pig fetuses and dissected along the greater curvature. The gastric mucosa was visually inspected, removed from the anterior wall, fixed in 10% formalin, embedded in paraffin, and cut into 5 μm sections. Primary antibodies against EPO-R and MUC5AC were used in the immunohistochemical staining. Tissue sections were incubated overnight with primary antibodies at 4 °C and then treated with horse radish peroxidase-labeled rabbit IgG antibody for 1hr at room temperature. The immunoreactive products were visualized with DAB staining (ABC method). Counterstaining was performed with hematoxylin. For negative controls, the primary antibody was replaced with rabbit or mouse nonimmune serum. Double staining for EPO-R and MUC5AC was performed with DAB staining (ABC method) followed by Ni-DAB staining (PAP method). For negative controls, the protocol was similar to that used for single staining.
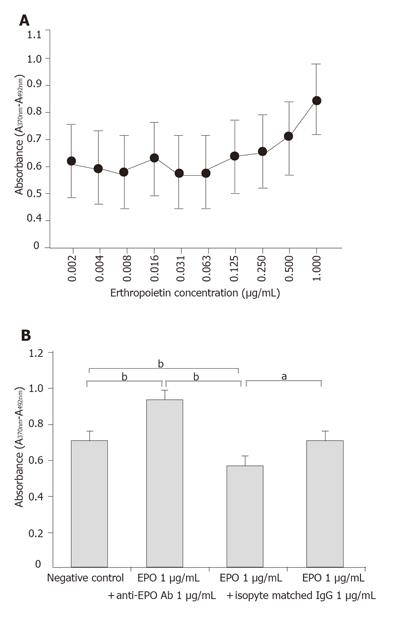
Cultured porcine gastric mucosal epithelial cells were incubated with Keratinocyte-SFM containing 20% newborn calf serum. EPO (0.002, 0.004, 0.008, 0.016, 0.031, 0.063, 0.125, 0.25, 0.5, 1 µg/mL) was added to the supernatant to evaluate the dose-dependent effects on growth potential of the gastric mucosal cells.
To evaluate the specificity of EPO effects, four sets of groups were established: 1) untreated cultures; 2) EPO 1 µg/mL treated cultures; 3) EPO 1µg/mL + anti-EPO antibody 1 µg/mL treated cultures; and 4) EPO 1 µg/mL +nonspecific antibody (rabbit IgG) 1 µg/mL treated cultures. Quantification of cell growth was measured by ELISA using BrdU.
Cultures and quantitative analysis were performed using 96-well plates (Nunc, Naperville, IL). Medium was prepared for each experimental group and aliquoted (50 µL/well) into 6 wells per group. The gastric mucosal epithelial cells were adjusted to a density of 1×105 cells/mL, and 50 µL were aliquoted into each well and incubated (5 000 cells/well) for 4 d. In accordance with a cell proliferation ELISA protocol, 10 µL/well of BrdU solution was added, and incubation was continued for 24 h. After discarding the supernatants, 200 µL/well of fixation reagent was added for 30 min to fix the cells and denature the DNA. After discarding the supernatants, 100 µL/well of anti-BrdU peroxidase solution was added and followed by incubation for 1 h. After discarding the supernatants and washing, 100 µL/well of substrate solution was added and incubated for 30 min, and absorbance at 370 nm (control wavelength, 492 nm) was assessed.
All results are expressed as mean ± SE. Statistical comparisons were made using the Student t-test or Welch’s t-test after the analysis of variances. The results were considered significantly different at P < 0.05.
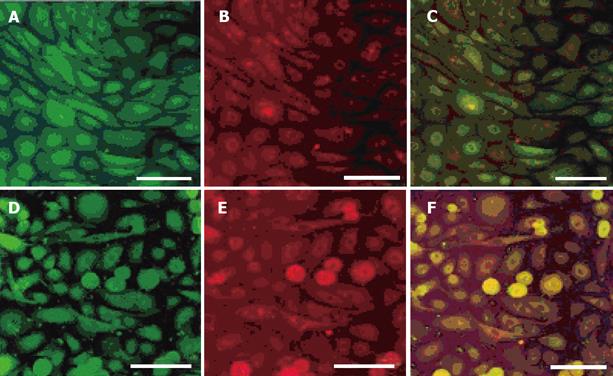
Cells were identified as gastric epithelial cells by double staining with anti-proton pump antibody, anti-pepsin antibody and anti-gastric mucin antibody in order to identify for gastric parietal cells, chief cells and mucin-producing cells, respectively (Figure 1). In the most cells, immunofluorescence showed double positive staining for anti-pepsin antibody and anti-proton pump antibody or for anti-pepsin antibody and anti-gastric mucin antibody.
RT-PCR: EPO-R was detected on the cultured porcine gastric mucosal epithelial cells. 207 bp products were detected (Figure 2).
We examined the expression of EPO-R protein in porcine epithelial cells by Western blotting. EPO-R protein expression was detected as a predicated size. The intensity of signals was dependent on anti-EPO-R antibody dilution (Figure 3).
EPO-R showed a punctate expression pattern at the base of gastric mucosal epithelial cells. MUC5AC was observed in the mucosal cell reticulum. Double staining was performed to examine the correlation between mucosal epithelial expression of EPO-R and presence of glycoproteins, which are an important component of the mucosal barrier. The findings were positive for EPO-R in the foveoral epithelial cells that were also positive for MUC5AC (Figure 4)
BrdU assay demonstrated a dose-dependent increase in growth potential of cultured porcine gastric mucosal epithelial cells at EPO doses ranging from 0.002 to 1 µg/mL. Evaluation of EPO dose dependence in this study showed the greatest growth potential at 1 µg/mL (Figure 5A) compared to untreated cultures, cultures treated with EPO showed statistically significant growth stimulation. In contrast, co-culturing with EPO antibody showed a significant growth inhibition effect. On the other hand, there was no significant inhibition effect of cell growth with a nonspecific antibody. These findings suggested that growth stimulation by EPO of gastric mucosal cells was mediated via EPO-specific receptors (Figure 5B).
The incidence of gastrointestinal bleeding is higher in hemodialysis patients than in the general population. The advent and use of histamine-2 (H2) receptor blockers and proton pump inhibitors (PPIs) has resulted in a small decrease in gastrointestinal bleeding events in hemodialysis patients, but the use of EPO therapy for treatment of anemia associated with renal failure has led to a more dramatic decrease in this complication for reasons that were previously unclear. Decreased blood supply secondary to anemia leads to low local oxygen concentrations in the gastric mucosa and results in formation of gastric lesions in dialysis patients[3,4,6]. Thus an increase in red blood cell production in response to EPO may reduce the incidence of hemorrhagic lesions by increasing oxygen delivery to the gastric mucosa. However, the present study suggests that the direct effect of EPO to gastric mucosa may also mediate the efficacy of EPO therapy found in dialysis patients.
The EPO gene was cloned and recombinant human EPO was purified in 1985, followed by identification of EPO-R on erythroid cells using iodine-labeled EPO. The EPO-R density on the surface of erythroid cells is low, ranging from a few hundred to a few thousand per cell. In the hematopoietic system, EPO-R is mainly expressed at the stage of late erythroid progenitor cells,[13] and the number of receptors decreases with differentiation into mature erythrocytes. In addition to erythroid progenitors, EPO-R is expressed on megakaryocytes[14], vascular smooth muscle cells[15], endothelial cells[7] skeletal myoblasts[16], neural cells[17], renal cells[18], ischemic retinal cells[19] and in the epicardium and pericardium[20]. Further, studies of the digestive tract have shown EPO-R mRNA in hepatic stromal cells in fetal mice[21], and EPO-R protein has been found in the intestinal villi during development in humans and rats.[22,23] Okada et al [10] investigated whether EPO stimulated gastric mucosal development in vitro in a rat gastric mucosal cell line (RGM-1) and reported that EPO produced a dose-dependent increase in thymidine uptake and c-myc gene expression. In addition, RT-PCR confirmed EPO-R gene expression, thus showing a direct growth effect on RGM-1 cells. It should be noted, however, that RGM-1 may represent transformed cell line rather than normal gastric epithelial cells. We previously demonstrated EPO-R mRNA expression in cultured porcine gastric mucosal microvascular endothelial cells[9].
In the present study, we confirmed the EPO-R mRNA in the cultured normal gastric epithelial cells. In addition, we used immunohistochemical analysis in the tissue sections to demonstrate EPO-R immunoreactivity in gastric mucosa cell, predominantly in foveolar epithelial cells. These data suggests that EPO may play a direct role in modulating normal gastric mucosal cells.
The dose-dependent effects of EPO on mucosal epithelial cell growth were observed at doses up to 1 µg/mL. Treatment of the porcine gastric mucosal epithelial cells with EPO clearly resulted in increased cell growth. The increase was inhibited by co-culturing with anti-EPO antibody, with significantly lower growth than in cells co-cultured with nonspecific antibody (rabbit IgG), which suggests that the growth stimulation effect of EPO is a direct and specific effect on the gastric mucosal epithelial cells.
EPO may have direct action on other types of mucosal and stromal cells. A previous study reported that treatment of rats with EPO before surgical anastomosis increased anastomosis strength and improved healing[24]. Further, in a retrospective study in premature rats, treatment with EPO decreased the incidence of experimentally-induced necrotizing enteritis[25,26]. In addition, treatment with EPO, both orally and parenterally, increased jejunal villus surface area. As compared to a control group, villus length was increased in groups treated with oral or parenteral EPO[27]. These findings indicate that EPO has a trophic action on the small intestine. EPO also stimulates growth of hepatic stromal cells in fetal mice, and furthermore, there is a dose-dependent effect. These results are consistent with data from the present study performed in gastric mucosal cells. EPO-specific mucosal cell growth is a noteworthy finding in our study, and the present study suggests EPO may prevent hemorrhagic gastric lesions in dialysis patients by a systemic hematopoietic effect and by a local effect on the gastric mucosa. EPO directly and specifically stimulated gastric mucosal epithelial cell proliferation.
S- Editor Guo SY L- Editor Elsevier HK E- Editor Cao L
| 2. | Bazan JF. A novel family of growth factor receptors: a common binding domain in the growth hormone, prolactin, erythropoietin and IL-6 receptors, and the p75 IL-2 receptor beta-chain. Biochem Biophys Res Commun. 1989;164:788-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 391] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 3. | Milito G, Taccone-Gallucci M, Brancaleone C, Nardi F, Filingeri V, Cesca D, Casciani CU. Assessment of the upper gastrointestinal tract in hemodialysis patients awaiting renal transplantation. Am J Gastroenterol. 1983;78:328-331. [PubMed] |
| 4. | Franzin G, Musola R, Mencarelli R. Morphological changes of the gastroduodenal mucosa in regular dialysis uraemic patients. Histopathology. 1982;6:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Tovbin D, Mazor D, Vorobiov M, Chaimovitz C, Meyerstein N. Induction of protein oxidation by intravenous iron in hemodialysis patients: role of inflammation. Am J Kidney Dis. 2002;40:1005-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Itoh K. The upper gastrointestinal disease state which was elucidated by erythropoietin administration (in Japanese). A Medincine Journal. 2003;39:148-155. |
| 7. | Anagnostou A, Liu Z, Steiner M, Chin K, Lee ES, Kessimian N, Noguchi CT. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci U S A. 1994;91:3974-3978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 438] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 8. | Carlini RG, Dusso AS, Obialo CI, Alvarez UM, Rothstein M. Recombinant human erythropoietin (rHuEPO) increases endothelin-1 release by endothelial cells. Kidney Int. 1993;43:1010-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 139] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Itoh K, Sawasaki Y, Takeuchi K. Effects of the erythropoietin medication on gastric hemorrhagic lesion in hemodialysis patients increased oxygen supply determined in vivo and growth stimulation of cultured gastric endothelial cells. Tokyo: Japanese Society for Microcirculation Publishers Inc, Nihon-Igakukan 2003; 85-86. |
| 10. | Okada A, Kinoshita Y, Maekawa T, Hassan MS, Kawanami C, Asahara M, Matsushima Y, Kishi K, Nakata H, Naribayashi Y. Erythropoietin stimulates proliferation of rat-cultured gastric mucosal cells. Digestion. 1996;57:328-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Jelkmann W, Wagner K. Beneficial and ominous aspects of the pleiotropic action of erythropoietin. Ann Hematol. 2004;83:673-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Takahashi K, Mitsui M, Takeuchi K, Uwabe Y, Kobayashi K, Sawasaki Y, Matsuoka T. Preservation of the characteristics of the cultured human type II alveolar epithelial cells. Lung. 2004;182:213-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Fraser JK, Lin FK, Berridge MV. Expression of high affinity receptors for erythropoietin on human bone marrow cells and on the human erythroleukemic cell line, HEL. Exp Hematol. 1988;16:836-842. [PubMed] |
| 14. | Fraser JK, Tan AS, Lin FK, Berridge MV. Expression of specific high-affinity binding sites for erythropoietin on rat and mouse megakaryocytes. Exp Hematol. 1989;17:10-16. [PubMed] |
| 15. | Ammarguellat F, Llovera M, Kelly PA, Goffin V. Low doses of EPO activate MAP kinases but not JAK2-STAT5 in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 2001;284:1031-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Ogilvie M, Yu X, Nicolas-Metral V, Pulido SM, Liu C, Ruegg UT, Noguchi CT. Erythropoietin stimulates proliferation and interferes with differentiation of myoblasts. J Biol Chem. 2000;275:39754-39761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 195] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Masuda S, Nagao M, Takahata K, Konishi Y, Gallyas F, Tabira T, Sasaki R. Functional erythropoietin receptor of the cells with neural characteristics. Comparison with receptor properties of erythroid cells. J Biol Chem. 1993;268:11208-11216. [PubMed] |
| 18. | Westenfelder C, Biddle DL, Baranowski RL. Human, rat, and mouse kidney cells express functional erythropoietin receptors. Kidney Int. 1999;55:808-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 181] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Junk AK, Mammis A, Savitz SI, Singh M, Roth S, Malhotra S, Rosenbaum PS, Cerami A, Brines M, Rosenbaum DM. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2002;99:10659-10664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 313] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 20. | Wu H, Lee SH, Gao J, Liu X, Iruela-Arispe ML. Inactivation of erythropoietin leads to defects in cardiac morphogenesis. Development. 1999;126:3597-3605. [PubMed] |
| 21. | Ohneda O, Yanai N, Obinata M. Erythropoietin as a mitogen for fetal liver stromal cells which support erythropoiesis. Exp Cell Res. 1993;208:327-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Juul SE, Yachnis AT, Christensen RD. Tissue distribution of erythropoietin and erythropoietin receptor in the developing human fetus. Early Hum Dev. 1998;52:235-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 224] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Juul SE, Joyce AE, Zhao Y, Ledbetter DJ. Why is erythropoietin present in human milk Studies of erythropoietin receptors on enterocytes of human and rat neonates. Pediatr Res. 1999;46:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Fatouros M, Dalekos GN, Mylonakis E, Vekinis G, Kappas AM. Alterations in body weight, breaking strength, and wound healing in Wistar rats treated pre- and postoperatively with erythropoietin or granulocyte macrophage-colony stimulating factor: evidence of a previously unknown anabolic effect of erythropoietin. J Lab Clin Med. 1999;133:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Ballin A, Bilker-Reich A, Arbel E, Davidovitz Y, Kohelet D. Erythropoietin, given enterally, stimulates erythropoiesis in premature infants. Lancet. 1999;353:1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Kanto WP, Hunter JE, Stoll BJ. Recognition and medical management of necrotizing enterocolitis. Clin Perinatol. 1994;21:335-346. [PubMed] |
| 27. | Juul SE, Ledbetter DJ, Joyce AE, Dame C, Christensen RD, Zhao Y, DeMarco V. Erythropoietin acts as a trophic factor in neonatal rat intestine. Gut. 2001;49:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |