Published online Jan 14, 2006. doi: 10.3748/wjg.v12.i2.228
Revised: June 8, 2005
Accepted: June 12, 2005
Published online: January 14, 2006
AIM: To determine whether biliary cirrhosis could induce pancreatic dysfunction such as modifications in endothelial nitric oxide synthase(eNOS) expression and whether the regulation of eNOS could be altered by the regulatory proteins caveolin and heat shock protein 90 (Hsp90), as well as by the modifications of calmodulin binding to eNOS.
METHODS: Immunoprecipitations and Western blotting analysis were performed in pancreas isolated from sham and cirrhotic rats.
RESULTS: Pancreatic injury was minor in cirrhotic rats but eNOS expression importantly decreased with the length (and the severity) of the disease. Because co-immunoprecipitation of eNOS with both Hsp90 and caveolin similarly decreased in cirrhotic rats, eNOS activity was not modified by this mechanism. In contrast, cirrhosis decreased the calmodulin binding to eNOS with a concomitant decrease in eNOS activity.
CONCLUSION: In biliary cirrhosis, pancreatic injury is minor but the pancreatic nitric oxide (NO) production is significantly decreased by two mechanisms: a decreased expression of the enzyme and a decreased binding of calmodulin to eNOS.
- Citation: Frossard JL, Quadri R, Hadengue A, Morel P, Pastor CM. Endothelial nitric oxide synthase regulation is altered in pancreas from cirrhotic rats. World J Gastroenterol 2006; 12(2): 228-233
- URL: https://www.wjgnet.com/1007-9327/full/v12/i2/228.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i2.228
Chronic bile duct ligation (BDL) in rats induces severe cirrhosis associated with extra-hepatic organ dysfunction in heart[1], lung[2,3], and extrahepatic vessels such as aorta[4,5] and mesenteric artery[6]. Chronic BDL also induces pulmonary injury associated with hypoxemia and pulmonary vasodilatation due to an excess production of endothelial nitric oxide (eNO)[2,3]. In aorta and mesenteric artery from BDL rats, hyporeactivity to vasoconstrictors also originates from NO overproduction[4-6]. Pancreas may suffer from hemodynamic consequences of cirrhosis, but information on eNOS expression and regulation in this splanchnic organ is missing.
In all types of cirrhotic livers, although eNOS protein expression is increased or decreased, the activity is uniformly decreased[7-10]. The reason for the decrease in hepatic NO bioavailability remains unclear. One explanation is that caveolin expression, a negative regulatory protein of eNOS, increases during cirrhosis and alters enzyme activity[10]. However, such negative post-translational regulation of eNOS is not unique.
In isolated endothelial cells, transcriptional and post-transcriptional mechanisms regulating eNOS have been described. although eNOS activity is associated with changes in Ca2+ concentrations within endothelial cells, increase in Ca2+ concentration alone is not sufficient to modify enzyme activity [11]. Binding of calmodulin (CaM) and flow of electrons from the reductase to the oxygenase domains of the enzyme are dependent on phosphorylation and dephosphorylation of various residues of eNOS. Additionally, proteins such as caveolin and heat shock protein 90 (Hsp90) associate with eNOS and modify the enzyme activity as well as its intracellular localization [12,13]. eNOS is co-localized with regulatory proteins and other molecules of the signal transduction pathway to form an “eNOS signaling complex” that modulates NO production [11]. For example, eNOS co-localizes with kinases that phosphorylate eNOS on the 495Thr residue with a concomitant decrease in enzyme activity [11,14,15].
The aim of our study was to determine whether biliary cirrhosis could induce pancreatic dysfunction including modifications in eNOS expression as observed in other extrahepatic organs and modify the regulation of eNOS by regulatory proteins and modifications in eNOS phosphorylation.
Sprague-Dawley rats were used in this study and divided into 3 groups, which underwent BDL 15, 30 and 60 days respectively before experiment and were designated as BDL-15, BDL-30 and BDL60 groups respectively.
Cirrhosis was induced 15, 30 and 60 days before the experiments. After laparotomy under 2-3% isoflurane anesthesia, Sprague-Dawley rats had a double ligation of the common bile duct with section between the two ligatures. The bile duct was ligated close to the liver to avoid pancreatic duct obstruction. Sham rats had laparotomy without bile duct ligation and were studied 15 days later. Normal rats with no laparotomy served as controls. The protocol was approved by the Animal Welfare Committee of the University of Geneva.
Tissues were collected from 15 rats (n = 3 in each group), and 3-µm thick paraffin sections were stained with hematoxylin and eosin as well as the Gomori technique.
Pancreatic tissues were homogenized in lysis buffer containing 50mM Tris HCl, 0.1mM EGTA, 0.1mM EDTA, 1% NP-40, 1% protease inhibitor cocktail, 1% phosphatase inhibitor, cocktails I and II (Sigma). Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting analysis were performed using minigels (BioRad, Switzerland). Protein extracts (100 µg) were separated on a 10% (eNOS and hsp90) or a 12% (caveolin and calmodulin) polyacrylamide gel. After the gel was transferred to a polyvinylidene difluoride membrane (Millipore, Volketswil, Switzerland), the membrane was blocked with PBS containing 5% nonfat dry milk (1 h at room temperature) and incubated overnight at 4°C with specific antibodies. The membrane was washed four times with PBS containing 0.2% Tween 20 and incubated for 1 h with an alkaline phosphatase-conjugated second antibody. After washed, protein was detected by chemiluminescence (Immune-Star, Bio-Rad) according to the manufacturer’s instructions. Molecular weight markers and positive controls were included for each experiment. Films were scanned using an ImageScanner densitometer equipped with the Labscan and Image Quant software (Amersham Biosciences). An equal quantity of protein stained with Ponceau Red was loaded on each line before the blocking was assessed.
Immunoprecipitation (IP) was performed by incubating 500 µg or 5 mg of protein extracts with antibodies directed to the native protein eNOS and the immune complexes were precipitated with protein G-sepharose beads (Sigma, Buchs, Switzerland). Complexes were washed twice in lysis buffer. The samples were boiled in loading buffer (Laemmli sample buffer, BioRad) to elute the bound proteins.Total or phosphorylated eNOS, Hsp90, caveolin and calmodulin were detected by Western blot. Antibodies used included mouse monoclonal anti-eNOS and rabbit polyclonal anti-caveolin-1 from BD Biosciences-Pharmingen (Basel, Switzerland), rat monoclonal anti-Hsp90 from Stressgen (Bioggio, Switzerland), mouse monoclonal anti-calmodulin from Upstate (Lake Placid, New-York) and goat polyclonal anti-phospho-eNOS-Thr495 from Santa Cruz Biotechnology (Santa Cruz, California).
Samples of pancreas (n = 3 in each group) were fixed in neutral buffered formalin for 24 h, processed and embedded in paraffin. Sections (3-µm thick) were cut and mounted on Superfrost/plus slides, then deparaffinized. The sections were treated with 1mM EDTA (pH = 8) to quench endogenous alkaline phosphatase activity. Non-specific binding was blocked by incubating sections in 1% BSA-Tris buffer for 30 min. Sections were then incubated with the primary antibody (rabbit polyclonal anti-caveolin-1 from BD Biosciences-Pharmingen) diluted in BSA-Tris buffer for 1 h. Control sections were incubated in BSA-Tris buffer without the first antibody and showed no immunostaining. After rinsing with Tris, the sections were incubated with alkaline phosphate-labeled polymer (DAKO Envision system) for 10 min and rinsed with Tris between each step. The antigen-antibody complex was visualized using the substrate chromogen solution containing 0.2 mM levamisole for 5-10 min. Sections were subsequently counterstained with Mayer’s hematoxylin solution for 2 min and rinsed in distilled water and then in 37 mM ammonia solution (10 times), rinsed in distilled water for 2 min and coverslipped with mounted media glycerol (DAKO). Images were obtained under Zeiss microscope (Zeiss, Zürich, Switzerland) using a Nikon COOLPIX995 camera and processed using the Coll view image and Photoshop sofware.
A similar technique was used to stain Hsp90 in pancreatic tissues with monoclonal mouse anti-Hsp90 (Stressgen) and eNOS with rabbit polyclonal anti-eNOS (BD Biosciences-Pharmingen). Monoclonal mouse anti-Hsp90 was incubated overnight.
Pancreatic cGMP was measured by competitive immun-oassay according to the instructions of the manufacturer (R&D systems, Wiesbaden-Nordenstadt, Germany).
Data for densitometric measurements were given as mean ± SD and difference between groups was determined with a Kruskal-Wallis or Mann-Whitney test for non-parametric variables. P < 0.05 was considered statistically significant.
Chronic bile duct ligation induced severe hepatic diseases as evidenced by the increase in serum bilirubin and aspartate aminotransferase concentrations (Table 1). In contrast, serum amylase concentration was slightly increased only in rats with BDL 60 d before the experiment.
| Sham | BDL-15 | BDL-30 | BDL-60 | ||
| ALAT (IU/L) | 34 ± 22 | 60 ± 43 | 76 ± 52 | 56 ± 11 | P = 0.55 |
| ASAT (IU/L) | 67 ± 40 | 194 ± 50 | 718 ± 328 | 262 ± 95 | P = 0.005 |
| Total bilirubin (µmol/L) | 5.3 ± 6.1 | 154.0 ± 9.2 | 120.7 ± 22.5 | 183.5 ± 28.1 | P < 0.0001 |
| Amylase (UI/L) | 1268 ± 113 | 1313 ± 115 | 1439 ± 293 | 2161 ± 299 | P = 0.019 |
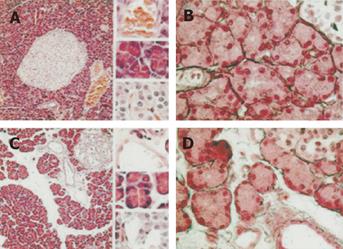
Pancreatic injury was minor in rats with BDL. On histological examinations, the only modification was the enlargement of the interstitial space in rats with BDL 60 days before the experiment (BDL-60 rats, Figure 1A).
No pancreatic necrosis was observed at any time point. The enlarged extracellular matrix consisted of edema because reticulin fibers surrounding acini in sham rats did not increase in BDL-60 rats (Figure 1B).
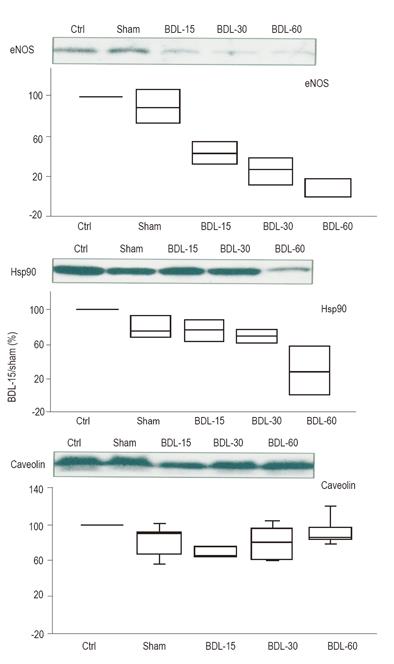
eNOS expression was significantly decreased in BDL-15, BDL-30 and BDL-60 rats (P = 0.003).
The two regulatory proteins of eNOS, Hsp90 (positive regulatory protein of eNOS) and caveolin (negative regulatory protein of eNOS), had different evolution. Hsp90 expression was maintained at the beginning of the disease but significantly decreased in BDL-60 rats (P = 0.02) while caveolin expression slightly decreased in BDL-15 rats but recovered in BDL-30 and BDL-60 rats (P = 0.05) (Figure 2).
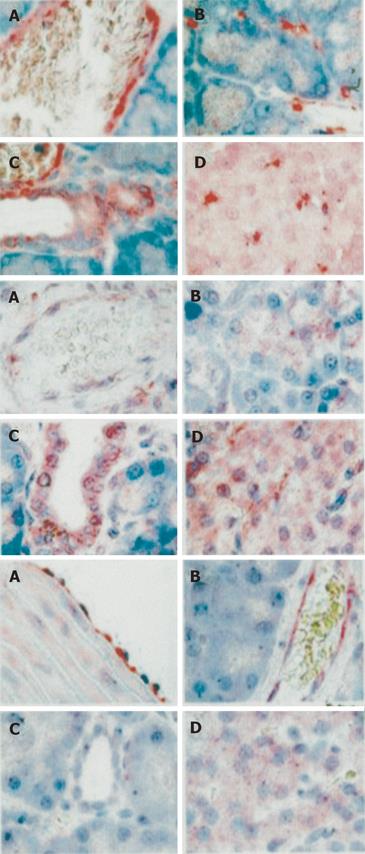
The localization of caveolin in pancreas is illustrated in Figure 3.
Caveolin was present in epithelial cells lining the pa-ncreatic ductules, cells within the islets of Langerhans and in the apex of acinar cells. Caveolin was also present in endothelial lining cells in veinules, microvessels surrounding acini and microvessels within Langerhans islets. Hsp90 was present in epithelial cells lining the pancreatic ductules, cells within the islets of Langerhans and in the apex of acinar cells. Hsp90 was also present in endothelial lining cells in veinules. Immunostaining of eNOS was weak in comparison to caveolin and Hsp90. Endothelial NOS was present only in endothelial cells and cells within the islets of Langerhans. In aorta isolated from BDL-60 rats (used as positive controls), eNOS was more easily detected.
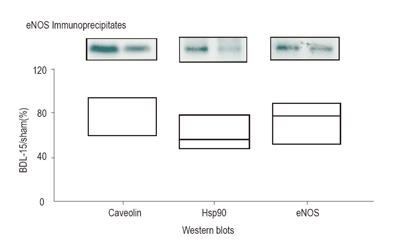
Hsp90 and caveolin were co-immunoprecipitated with eNOS in pancreas isolated from sham and BDL-15 rats (Figure 4).
Because in BDL-15 rats, the reduction in coimmun-oprecipitation of Hsp90 and caveolin was similar to the decrease in eNOS expression (P = 0.74), eNOS activity was not modified by this mechanism in BDL-15 rats. Co-immunoprecipitation from pancreas isolated from BDL-30 and BDL-60 rats was not available because the amount of eNOS protein in samples was too low.
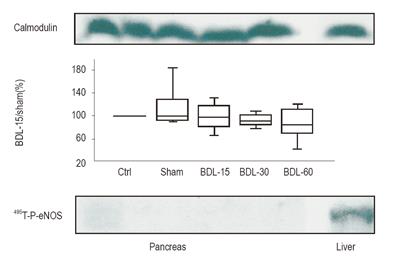
In pancreatic lysates, 495Thr-P eNOS was not detectable at any time point, though the phosphorylated eNOS was easily detected in the liver (Figure 5).
Expression of CaM was evident and did not differ between groups (P = 0.95). In BDL-15 rats, the co-immunoprecipitation of eNOS with CaM decreased while 495Thr-P eNOS became detectable.
Interestingly, the cGMP concentration in pancreatic tissue was slightly lower in BDL-15 rats than in sham rats (62 pmol/g vs 90 pmol/g protein, P = 0.086).
Our study showed that pancreatic injury was mild in rats with chronic biliary cirrhosis. Nevertheless, the expression of eNOS importantly decreased with the duration (and the severity) of the disease. The regulation of eNOS by Hsp90 and caveolin was not modified by a 15-day BDL but cirrhosis decreased the CaM binding to eNOS with a concomitant phosphorylation of the enzyme on 495Thr residue, suggesting that the activity of the enzyme decreases through this regulatory pathway. Consequently, besides liver, lungs and extrahepatic vessels, pancreas is another organ in which eNOS regulation is markedly impaired by cirrhosis.
BDL is frequently used to induce chronic biliary cirrhosis in rats. In this model, the severity of hepatic injury increases with the duration of BDL. This model is also characterized by extra-hepatic injuries. Chronic BDL is associated with severe cardiomyopathy[1].Vasoconstriction to phenylephrine or vasodilatation to acetylcholine decreases in large vessels such as aorta[5,16,17], mesenteric[18] and pulmonary arteries[3]. Additionally, the modifications observed in lungs from BDL rats mimic the hepatopulmonary syndrome that complicates human cirrhosis [3]. In the present study, we described for the first time a slight pancreatic injury that appeared only 2 months after BDL.
Organ dysfunction during biliary cirrhosis includes modification in NO release. eNOS expression has been investigated in livers from normal and BDL rats and the expression is similar in control and BDL-24 rats[19]. Modifications in eNOS expression have also been found in extrahepatic organs isolated from rats with BDL. For example, in aortic rings isolated from BDL-28 rats, eNOS protein expression is increased. For example, in aortic rings isolated from BDL-28 rats, eNOS protein expression is increased[20]. These results have been confirmed in rats sacrificed at different time points (BDL-21 and BDL-30 rats)[5,21]. The expression of eNOS in pulmonary arteries isolated from BDL-28 rats is also increased [3,22]. The high staining of eNOS observed in aorta isolated from BDL-60 rats (Figure 3) might confirm these findings.
In our study, we used the BDL model of cirrhosis. However, additional experimental models exist, including chronic ingestion of CCl4 that also shows a dysregulation of eNOS[8]. Though BDL rats develop hepatic cirrhosis with systemic vasodilatation and hepatopulmonary syndrome as observed in humans[3], the results obtained in BDL rats should be extrapolated to humans with cautions.
eNOS has already been detected in normal pancreas[23-25]. For the first time, we showed that eNOS expression significantly decreased in pancreas collected from BDL-15 rats with a further decrease associated with the duration of bile duct ligation. Interestingly, we localized eNOS in endothelial cells and cells within the islets of Langerhans. However, the staining was weak, suggesting that eNOS has a low expression in native pancreas.
The regulation of eNOS is a process determined by a cascade of events, including changes in (1) NOS mRNA and protein levels, (2) association of eNOS with regulatory proteins in the signaling complex, (3) changes in intracellular location of the enzyme and (4) phosphorylation of serine, threonine and tyrosine residues. Though many of these steps have been relatively well elucidated in in vitro models and in cell lines overexpressing one or more components of the signaling complex, much more work is required to determine which modification plays a dominant role in the regulation of eNOS activity in native tissues.
In our experimental study, the two regulatory proteins of eNOS, Hsp90 and caveolin, had a different evolution. Caveolin expression slightly decreased in BDL-15 rats but recovered in BDL-30 and BDL-60 rats. The reason for the transient decrease is unclear. In contrast, in livers from BDL rats, caveolin expression increases [10]. In normal pancreas, caveolin is detectable in membrane preparations [26]. Caveolin-1 has been identified in exocrine cells and secretagogues known to stimulate secretions in exocrine cells also release caveolin [27]. However, the function of secreted caveolin in pancreas is unknown. We also identified caveolin in epithelial cells lining the pancreatic ductules, cells within the islets of Langerhans, endothelial cells lining veinules, microvessels surrounding acini and inside Langerhans islets.
In our study, Hsp90 expression was maintained at the beginning of the disease but significantly decreased in BDL-60 rats. Hsp90 is constitutively expressed at high concentration in normal pancreas and the expression does not increase during acute pancreatitis [28] or hyperthermia [29,30]. In the present study, we showed for the first time, the localization of Hsp90 in epithelial cells lining the pancreatic ductules, cells within the islets of Langerhans, acinar cells and endothelial cells in veinules.
In BDL livers, co-immunoprecipitation of caveolin with eNOS increases and this binding is associated with a decreased eNOS activity[10]. Co-immunoprecipitation of Hsp90 with eNOS was not investigated in this study. In pancreas from BDL-15 rats, the co-immunoprecipitation of eNOS with both caveolin and Hsp90 similarly decreased, suggesting that the two regulatory proteins have no effect on pancreatic eNOS activity.
Phosphorylation of eNOS on the 495Thr residue in isolated endothelial cells is associated with a decrease in enzyme activity. The link between phosphorylation and NO production can be explained by the interference with the binding of CaM to the CaM-binding domain of eNOS [11,14,15]. Changes in 495Thr phosphorylation are generally associated with stimuli such as bradykinin, histamine and Ca2+ ionophores. In endothelial cells, these Ca2+ elevating agonists decrease 495Thr phosphorylation of eNOS with a concomitant increased binding of CaM to the CaM-binding site of eNOS.
Interestingly, we showed a similar regulatory mechanism induced by cirrhosis in native pancreas. The495Thr- phosphorylated form of eNOS undetectable in normal pancreas (in contrast to normal liver) appeared in pancreas from cirrhotic rats. Concomitantly, the increased expression of 495Thr-P-eNOS was associated with a decreased binding of CaM to eNOS.
Besides the regulatory proteins and CaM, other proteins are likely to interfere with the regulation of eNOS in the pancreas [13]. Phosphorylation of 1179Ser is another well-identified mechanism activating the enzyme. These regulatory pathways and probably many others concomitantly modify eNOS activity. Although we found a decreased cGMP concentration in pancreas from BDL-15 rats, the numerous pathways regulating eNOS preclude the modifications of eNOS activity to the decreased binding of CaM.
In conclusion, co-immunoprecipitation of Hsp90 and caveolin with eNOS has no effect on eNOS activity while the binding of CaM to eNOS decreases with a likely concomitant decrease in eNOS activity. Besides liver, lungs and extra-hepatic vessels, pancreas is another organ with its eNOS expression modified by cirrhosis.
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Wu M
| 1. | Liu H, Song D, Lee SS. Role of heme oxygenase-carbon monoxide pathway in pathogenesis of cirrhotic cardiomyopathy in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;280:G68-G74. [PubMed] |
| 2. | Fallon MB, Abrams GA, McGrath JW, Hou Z, Luo B. Common bile duct ligation in the rat: a model of intrapulmonary vasodilatation and hepatopulmonary syndrome. Am J Physiol. 1997;272:G779-G784. [PubMed] |
| 3. | Fallon MB, Abrams GA, Luo B, Hou Z, Dai J, Ku DD. The role of endothelial nitric oxide synthase in the pathogenesis of a rat model of hepatopulmonary syndrome. Gastroenterology. 1997;113:606-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 153] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Tazi KA, Moreau R, Heller J, Poirel O, Lebrec D. Changes in protein kinase C isoforms in association with vascular hyporeactivity in cirrhotic rat aortas. Gastroenterology. 2000;119:201-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Tazi KA, Barrière E, Moreau R, Heller J, Sogni P, Pateron D, Poirel O, Lebrec D. Role of shear stress in aortic eNOS up-regulation in rats with biliary cirrhosis. Gastroenterology. 2002;122:1869-1877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Namiranian K, Samini M, Mehr SE, Gaskari SA, Rastegar H, Homayoun H, Dehpour AR. Mesenteric vascular bed responsiveness in bile duct-ligated rats: roles of opioid and nitric oxide systems. Eur J Pharmacol. 2001;423:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Shah V, Toruner M, Haddad F, Cadelina G, Papapetropoulos A, Choo K, Sessa WC, Groszmann RJ. Impaired endothelial nitric oxide synthase activity associated with enhanced caveolin binding in experimental cirrhosis in the rat. Gastroenterology. 1999;117:1222-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 233] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 268] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 9. | Gupta TK, Toruner M, Chung MK, Groszmann RJ. Endothelial dysfunction and decreased production of nitric oxide in the intrahepatic microcirculation of cirrhotic rats. Hepatology. 1998;28:926-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 261] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Shah V, Cao S, Hendrickson H, Yao J, Katusic ZS. Regulation of hepatic eNOS by caveolin and calmodulin after bile duct ligation in rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1209-G1216. [PubMed] |
| 11. | Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1-12. [PubMed] |
| 12. | Balligand JL. Heat shock protein 90 in endothelial nitric oxide synthase signaling: following the lead(er). Circ Res. 2002;90:838-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Kone BC. Protein-protein interactions controlling nitric oxide synthases. Acta Physiol Scand. 2000;168:27-31. [PubMed] |
| 14. | Venema RC. Post-translational mechanisms of endothelial nitric oxide synthase regulation by bradykinin. Int Immunopharmacol. 2002;2:1755-1762. [PubMed] |
| 15. | Harris MB, Ju H, Venema VJ, Liang H, Zou R, Michell BJ, Chen ZP, Kemp BE, Venema RC. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem. 2001;276:16587-16591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 292] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 16. | Pateron D, Oberti F, Lefilliatre P, Veal N, Tazi KA, Sogni P, Poirel O, Heller J, Moreau R, Cales P. Relationship between vascular reactivity in vitro and blood flows in rats with cirrhosis. Clin Sci (Lond). 1999;97:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Rastegar H, Jorjani M, Roushanzamir F, Ahmadiani A, Namiranian K, Dehpour AR. Time-dependent reduction of acetylcholine-induced relaxation in aortic rings of cholestatic rats. Pharmacol Res. 2001;44:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Nadal FJ, Atucha NM, Iyu D, García-Estañ J. Interaction of nitric oxide with calcium in the mesenteric bed of bile duct-ligated rats. Br J Pharmacol. 2002;135:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Wei CL, Khoo HE, Lee KH, Hon WM. Differential expression and localization of nitric oxide synthases in cirrhotic livers of bile duct-ligated rats. Nitric Oxide. 2002;7:91-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Liu H, Song D, Lee SS. Increased nitric oxide synthase expression in aorta of cirrhotic rats. Life Sci. 1999;64:1753-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Schmandra TC, Folz IC, Kimpel M, Fleming I, Holzer K, Hanisch EW. Cirrhosis serum induces a nitric oxide-associated vascular hyporeactivity of aortic segments from healthy rats in vitro. Eur J Gastroenterol Hepatol. 2001;13:957-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Zhang J, Ling Y, Luo B, Tang L, Ryter SW, Stockard CR, Grizzle WE, Fallon MB. Analysis of pulmonary heme oxygenase-1 and nitric oxide synthase alterations in experimental hepatopulmonary syndrome. Gastroenterology. 2003;125:1441-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Takács T, Czakó L, Morschl E, László F, Tiszlavicz L, Rakonczay Z, Lonovics J. The role of nitric oxide in edema formation in L-arginine-induced acute pancreatitis. Pancreas. 2002;25:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Kawaguchi M, Koshimura K, Sohmiya M, Murakami Y, Gonda T, Kato Y. Effect of insulin on nitric oxide synthase-like immunostaining of arteries in various organs in Zucker diabetic fatty rats. Eur J Endocrinol. 2001;145:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Akesson B, Henningsson R, Salehi A, Lundquist I. Islet constitutive nitric oxide synthase and glucose regulation of insulin release in mice. J Endocrinol. 1999;163:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Parker EM, Zaman MM, Freedman SD. GP2, a GPI-anchored protein in the apical plasma membrane of the pancreatic acinar cell, co-immunoprecipitates with src kinases and caveolin. Pancreas. 2000;21:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Liu P, Li WP, Machleidt T, Anderson RG. Identification of caveolin-1 in lipoprotein particles secreted by exocrine cells. Nat Cell Biol. 1999;1:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Ethridge RT, Ehlers RA, Hellmich MR, Rajaraman S, Evers BM. Acute pancreatitis results in induction of heat shock proteins 70 and 27 and heat shock factor-1. Pancreas. 2000;21:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Tashiro M, Ernst SA, Edwards J, Williams JA. Hyperthermia induces multiple pancreatic heat shock proteins and protects against subsequent arginine-induced acute pancreatitis in rats. Digestion. 2002;65:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Yamagishi N, Nakayama K, Wakatsuki T, Hatayama T. Characteristic changes of stress protein expression in streptozotocin-induced diabetic rats. Life Sci. 2001;69:2603-2609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |