Published online Apr 7, 2006. doi: 10.3748/wjg.v12.i13.2120
Revised: November 11, 2005
Accepted: November 18, 2005
Published online: April 7, 2006
AIM: To explore the effect of hypobaric hypoxia on mitochondrial energy metabolism in rat liver.
METHODS: Adult male Wistar rats were exposed to a hypobaric chamber simulating 5000 m high altitude for 23 h every day for 0 (H0), 1 (H1), 5 (H5), 15 (H15) and 30 d (H30) respectively. Rats were sacrificed by decapitation and liver was removed. Liver mitochondria were isolated by differential centrifugation program. The size of adenine nucleotide pool (ATP, ADP, and AMP) in tissue and mitochondria was separated and measured by high performance liquid chromatography (HPLC). The adenine nucleotide transporter (ANT) activity was determined by isotopic technique. The ANT total protein level was determined by Western blot.
RESULTS: Compared with H0 group, intra-mitochondrial ATP content decreased in all hypoxia groups. However, the H5 group reached the lowest point (70.6%) (P < 0.01) when compared to the control group. Intra-mitochondrial ADP and AMP level showed similar change in all hypoxia groups and were significantly lower than that in H0 group. In addition, extra-mitochondrial ATP and ADP content decreased significantly in all hypoxia groups. Furthermore, extra-mitochondrial AMP in groups H5, H15 and H30 was significantly lower than that in H0 group, whereas H1 group had no marked change compared to the control situation. The activity of ANT in hypoxia groups decreased significantly, which was the lowest in H5 group (55.7%) (P < 0.01) when compared to H0 group. ANT activity in H30 group was higher than in H15 group, but still lower than that in H0 group. ANT protein level in H5, H15, H30 groups, compared with H0 group decreased significantly, which in H5 group was the lowest, being 27.1% of that in H0 group (P < 0.01). ANT protein level in H30 group was higher than in H15 group, but still lower than in H0 group.
CONCLUSION: Hypobaric hypoxia decreases the mitochondrial ATP content in rat liver, while mitochondrial ATP level recovers during long-term hypoxia exposure. The lower level of extra-mitochondrial ATP may be related to the decrease of ANT activity during hypoxia exposure.
- Citation: Li CY, Liu JZ, Wu LP, Li B, Chen LF. Effects of hypobaric hypoxia on adenine nucleotide pools, adenine nucleotide transporter activity and protein expression in rat liver. World J Gastroenterol 2006; 12(13): 2120-2124
- URL: https://www.wjgnet.com/1007-9327/full/v12/i13/2120.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i13.2120
The liver is the largest metabolic organ in the body. It performs a number of important and complex biological functions that are essential for survival. It also plays important roles in metabolism of carbohydrates, proteins, lipids, drugs, as well as in bile formation and secretion. Energy metabolism is closely related to these normal functions of liver. Mitochondria are the “energy factory” of cells. The adenine nucleotide transporter (ANT) is the most integral protein in inner mitochondrial membrane and consists of two identical subunits of 32 KD[1]. It catalyzes the transporter of cytosolic ADP and mitochondrial ATP in the process of phosphorylation[1]. Consequently, ANT is an important link between the cytosolic energy consumption and mitochondrial energy process yield[1,2]. Hypoxia could influence mitochondrial oxygenation respiration function[3,4] and F0-F1 ATPase activity of rat brain[4]. However, little is known about the relationship between the effects of hypobaric hypoxia on mitochondrial energy metabolism changes and ANT function in rat liver exposed to hypoxia. We therefore used HPLC, isotopic assay and Western blot to examine the inner- and extra-mitochondria adenine nucleotide pool, ANT activity and its total protein level of rat liver exposed to hypobaric hypoxia.
[2,8-3H] ADP was obtained from Perkin-Elmer. Atractyloside (ATR), adenosine-5’-diphosphoric acid (ADP), albumin bovine serum (BSA), nitroblue tetrazolium chloride (NBT), 5-bromo-4-chloro-3-indolye-phosphate (BCIP) and mouse anti-goat IgG-alkaline phosphatase (IgG-AP) were supplied by Sigma. Goat anti-human ANT polyclonal antibody was purchased from Santa Cruz.
Adult male Wistar rats (150-200 g) were used in the experiments. The rats were exposed to a hypobaric chamber simulating 5 000 m high altitude for 23 h every day for 0 (H0), 1 (H1), 5 (H5), 15 (H15) and 30 d (H30) respectively. Animals were fed with laboratory chow and tap water ad libitum. Rats were sacrificed by decapitation at seal level (H0 group) and hypobaric chamber simulating 4000 m high altitude (H1, H5, H15, H30 groups). After decapitation the liver tissues were immediately excised, and part of it was frozen in liquid nitrogen, and then transferred to -70 °C for storage until use. The remaining liver tissues were rapidly placed into ice-cold isolation medium [0.25 mol/L sucrose, 10 mmol/L 4-(2-hydroxymethyl)-1-piperazine ethane sulfonic acid (HEPES), 1 mmol/L ethylene diamine tetraacetic acid (EDTA), pH 7.4][5]. The tissues were chopped finely with scissors while being washed three times with ice-cold isolation medium and then manually homogenized by 15 up and down strokes with Teflon glass pestle. The liver mitochondria were isolated by centrifugation as established in our laboratory[3,4]. The protein content in the mitochondria suspension was assayed by Lowry’s method using BSA as the standard.
Adenine nucleotides were separated and quantitated by HPLC[4,6]. Briefly, 500 mmol/L ice-cold HClO4 was added to 200 μL liver mitochondria suspension and after 5 min incubation at 4 °C, the liquid was centrifuged (12 000 r/min, 20 min) at 4 °C and the supernatant was saved and neutralized with 1 mol/L K2CO3 to pH 6.5-7.0. The liquid was centrifuged (12 000 r/min, 10 min) at 4 °C again. The samples were maintained frozen at -70 °C until defrosted and analysed by HPLC equipped with high pressure pumps (Waters, USA), fit with 4.6 mm× 250 mm hypersil 18 (5 μm pore size, from Sigma). The samples were applied in a total volume of 20 μL. The results were shown as nmol adenine nucleotides/mg mitochondrial protein.
The liver tissues stored at -70 °C were rapidly transferred into ice-cold 0.5 mol/L HClO4, chopped finely with scissors, and then manually homogenized by 10 up and down strokes with Teflon glass pestle. The homogenate was placed at 4°C for 5 min, centrifuged (12 000 r/min, 20 min) at 4 °C. The supernatant was saved and neutralized with 1 mol/L K2CO3 to pH 6.5-7.0. The liquid was centrifuged (12 000 r/min, 10 min) at 4 °C again and maintained frozen at -70 °C until supernatant was analyzed by HPLC as described above. The results were expressed as nmol/mg tissue.
The activity of ANT was determined at 4°C by isotopic technique[1]. 3H- ADP label was stopped by atractyloside, a specific inhibitor of ANT. Fifty microliter mitochondrial suspension solution was diluted by 150 μL ice-cold isolation medium. Twenty microliter 3H- ADP (specific activity 33.9 Ci/mmol ADP) 0.3 μmol/L were added. After 10 s incubation at 4 °C, the reaction was inhibited by 50 μL 3.2 nmol/L ATR, then centrifuged (12 000 r/min, 20 min) at 4 °C. The precipitation was dissolved with 1 ml ice-cold isolation medium, centrifuged (12 000 r/min, 20 min) at 4°C again for 20 min and the process was repeated three times. The pellet was digested at 70 °C with 7 mol/L HClO4 200 μL, 8.8 mol/L H2O2 400 μL for 40 min. The 200 μL sample was dissolved in 1 ml scintillator(5 g PPO was dissolved by 700 mL dimethylbenzene and 300 mL anhydro-ethanol)and measured in liquid scintillation counter. ANT activity was calculated from the radioactivities of H3-ADP [count per min (cpm)]. Nonspecific binding of H3-ADP to mitochondria was determined by incubation of mitochondrial samples with 50 μL 3.2 nmol/L ATR prior to addition of 0.3 μmol/L 3H- ADP. The results were expressed as pmol ADP/min per milligram mitochondrial protein.
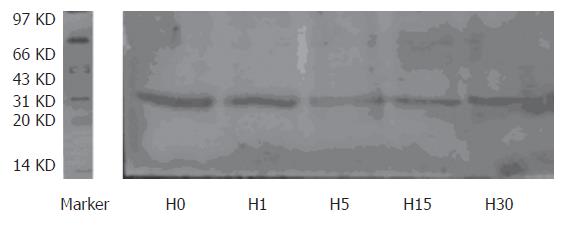
The rat liver mitochondrial ANT protein level was determined by Western blot[1]. The mitochondria sample was solubilized in sample buffer and supplemented with 5 μL β-mercaptoethanol. Following that, it was heated for 10 min at 100 °C and supplemented with 5 μL β-mercaptoethanol again. Electrophoresis was performed on a 12% polyacrylamide slab gel with Tris/glycine running buffer. The staking gel contained 5% polyacrylamide. The lanes were loaded with 30 μL aliquots of solubilized mitochondria (10 μg). After separation the protein bands were stained with Commassie blue for 4 h or transferred to polyvinylidene difluoride (PVDF) sheet for 90 min. The PVDF sheet was washed twice with Tris-buffered saline (TBS) containing 0.1% Tween-20 (0.1% TBST), pH 7.4 at room temperature. ANT polyclonal antibody at a 1:1000 dilution was used. Incubation was performed in 0.1% TBST, pH 7.4 for at least 12 h at 4°C. The sheet was washed six times with 0.1% TBST at room temperature. Mouse anti-goat IgG coupled with alkaline phosphatase at a 1:1000 dilution was used as secondary antibody. Incubation was performed in 0.1% TBST, pH 7.4 for 1 h at room temperature. The sheet was washed six times with 0.1% TBST again. Antigen was visualized by luminescence (NBT/BCIP). Signals were quantified with Smartview (Furi Science and Technology Co., Ltd., Shanghai, China).
Results were expressed as mean ± SD. Significant difference was determined by one-way ANOVA followed by LSD test between different groups. Statistical analyses were performed using SPSS 12.0 software.
Compared with H0 group, liver tissue ATP and ADP content decreased significantly in all hypoxia groups (P <0.01). Tissue AMP in groups H5, H15, and H30 was significantly lower than in H0 group (P < 0.05 and P < 0.01), while H1 group had no marked change compared to H0 group (Table 1).
| Group | n | AMP | ADP | ATP |
| H0 | 6 | 9.32 ± 1.63 | 9.02 ± 0.87 | 4.00 ± 0.26 |
| H1 | 6 | 7.14 ± 1.41 | 5.53 ± 0.71b | 2.40 ± 0.29b |
| H5 | 6 | 4.90 ± 0.74b | 4.57 ± 0.48b | 2.41 ± 0.17b |
| H15 | 6 | 6.08 ± 1.57a | 5.40 ± 1.31b | 3.11 ± 0.25b |
| H30 | 6 | 6.58 ± 1.19a | 6.21 ± 1.36b | 3.27 ± 0.30b |
Compared with H0 group, intra-mitochondrial ATP content decreased in all hypoxia groups, which in H1 group was 70.6% of that in H0 group (P < 0.01) reaching the lowest point. Intra-mitochondrial ADP and AMP levels showed the same change and were significantly lower in H0 group than that in all hypoxia groups (P < 0.01) (Table 2).
| Group | n | AMP | ADP | ATP |
| H0 | 6 | 6.84 ± 1.57 | 11.34 ± 1.97 | 5.47 ± 0.54 |
| H1 | 6 | 3.68 ± 0.42b | 4.52 ± 1.07b | 3.86 ± 0.20b |
| H5 | 6 | 2.72 ± 1.09b | 4.76 ± 1.56b | 4.03 ± 0.25b |
| H15 | 6 | 3.94 ± 0.68b | 5.07 ± 0.10b | 4.50 ± 0.35b |
| H30 | 6 | 3.77 ± 0.67b | 8.70 ± 2.11b | 4.72 ± 0.60a |
Compared with H0 group, extra-mitochondrial ATP and ADP content decreased significantly in all hypoxia groups (P <0.05 and P <0.01). Extra-mitochondrial AMP in groups H5, H15, and H30 was significantly lower than that in H0 group (P <0.01), while in H1 group it had no marked change compared with H0 group (Table 3).
| Group | n | AMP | ADP | ATP |
| H0 | 6 | 8.64 ± 1.58 | 8.08 ± 0.93 | 3.45 ± 0.22 |
| H1 | 6 | 6.77 ± 1.40 | 5.07 ± 0.74b | 2.01 ± 0.27b |
| H5 | 6 | 4.63 ± 0.82b | 4.09 ± 0.60b | 2.01 ± 0.15b |
| H15 | 6 | 5.68 ± 1.60a | 4.90 ± 1.34b | 2.67 ± 0.27b |
| H30 | 6 | 6.21 ± 2.01a | 5.37 ± 1.53b | 2.80 ± 0.33a |
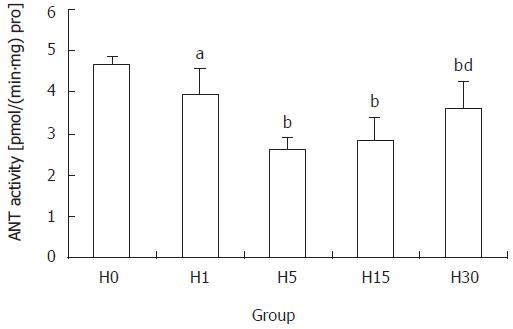
Compared with H0 group, the activity of ANT decreased significantly in all hypoxia groups, which in H5 group was 55.7% of that in H0 group (P < 0.01), being the lowest. Activity in H30 group was higher than that in H15 group (P <0.01), but was still lower than in H0 group (P < 0.01) (Figure 1).
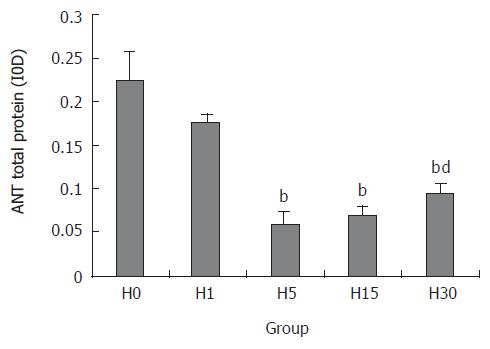
ANT protein expression in H5, H15, and H30 groups, compared with H0 group, decreased significantly, which in H5 group was the lowest point (27.1%) (P < 0.01). The expression in H30 group was higher than that in H15 group (P < 0.01), but was still lower than that in H0 group (P < 0.01) (Figures 2 and 3).
ATP is the direct energy for cell usage. Mitochondrial ATP level is influenced by two factors. First, mitochondrial ATP is produced by oxidative phosphorylation. Mitochondria oxidative respiration and phosphorylation states are the main factors that affect the ATP level. Second, mitochondrial ATP provides energy for cytoplasm as well as for its own demand such as the synthesis of mitochondrial DNA, RNA and proteins, etc. Our previous work showed that during hypoxia exposure, mitochondrial ATP content and F0-F1 ATPase activity of rat brain decreased significantly compared with control [4,7]. This indicates that hypoxia could influence ATP production and then the mitochondrial ATP level. However, there were no reports about the hypobaric hypoxia effect on rat liver mitochondrial adenine nucleotide pool.
The current results showed that ATP level in rat liver mitochondria reduced during hypoxia exposure, which in H1 group was the lowest point, 70.6% of control (P < 0.01). The decrease of mitochondrial ATP content may be related to the following factors. First, mitochondrial oxidative respiratory function was inhibited. Our previous study showed that hypobaric hypoxia inhibited the oxidative respiratory function of mitochondria in rat brain[3,4,6]. Our results (data not shown) also revealed that hypoxia significantly decreased the mitochondrial three state oxygen consumption and respiratory control rate in rat liver, while significantly increased the rat liver mitochondrial four state oxygen consumption. Second, hypoxia decreased the mitochondrial membrane potential (MMP). Our results (data not shown) demonstrated that hypoxia inhibited the MMP of rat hepatocytes. However, MMP is the motive power of mitochondrial ATP synthesis. Thirdly, hypoxia lowered the mitochondrial F0-F1 ATPase activity[4,8]. Fourthly, the lower mitochondrial ATP content may be also related to the reduction of intra-mitochondrial ADP concentration[1], intra-mitochondrial Ca2+ content[9] and extra-mitochondrial adenine nucleotide pool[10,11] .
ANT is the most integral protein in inner mitochondrial membrane and consists of two identical subunits of 32 KD[1]. It is a key energy link between the mitochondria and cytoplasm since it catalyses the transmembrane exchange between ATP synthesized by the F1-Fo ATP synthase inside mitochondria and ADP generated by the metabolism in cytoplasm[1,6]. The ADP/ATP exchange follows the Michaelis-Menten kinetics and ANT activity is moderate, 1500-2000 molecules per min[12]. Schonfeld et al reported that matrix adenine nucleotides and the ANT protein content are associated with the changes of the ANT activity in rat heart mitochondria[1]. Rulfs et al also reported that matrix adenine nucleotide concentration influenced the ANT activity in rabbit liver mitochondria[13]. However, little is known about the effect of hypobaric hypoxia on mitochondrial ANT activity in rat liver.
Our study showed that the activity of ANT after hypoxia exposure decreased significantly. The ANT activity was the lowest point, 55.7% of control after hypoxia exposure for 5 d (P < 0.01), while after hypoxia exposure for 30 d it was higher than after 15 d exposure (P < 0.01), but was still lower than control. This indicates that hypoxia could inhibit the mitochondrial ANT activity in rat liver. The decrease of ANT activity may be related to the following factors. First, the ANT protein level and content was the main factor. Our results showed that ANT protein expression decreased significantly in H5, H15, H30 groups, which in H5 group was 27.1% of that in H0 group. Second, MMP also influenced the ANT activity[14]. Passarella et al reported that helium neon laser increased the rate of ADP/ATP exchange through increasing the MMP in rat liver[15]. In the presence of an MMP of about 100 mV positive inside, the rates of the [14C] ATPout/ADPin exchanges were stimulated[3]. All these indicate that MMP is one of the most important factors that affect the ANT activity. The mechanism that decrease of MMP reduced ANT activity is not clear. Thirdly, the change of ANT conformation also influenced its activity. ANT has two conformational states, cytosolic conformation (c-confromation) and matrix conformation (m-conformation)[16,17]. ANT is not a pore, which opens or closes simply as a response to stimuli. Conformational changes have to occur to release nucleotides to the matrix (and the reverse) without creating leakage in the membrane. Fourthly, it was reported that the size of mitochondrial adenine nucleotide pool influenced the ANT activity. Previous studies showed that the postnatal increase in the matrix adenine nucleotides concentration contributed to the increase of ANT activity in rat liver[13] and heart[1].
The decrease of extra-mitochondrial ATP level influences the mitochondrial carrier family including ANT synthesis and transport. Extra-mitochondrial ATP level is mainly determined by the ANT activity. However, the lower ANT protein level has an identical role in influencing ANT activity during hypoxia. So the ANT activity-ANT protein level- ATP content form the vicious cycle and aggravate the dysfunction of cell energy metabolism during hypoxia exposure.
S- Editor Wang J L- Editor Zhu LH E- Editor Ma WH
| 1. | Schönfeld P, Schild L, Bohnensack R. Expression of the ADP/ATP carrier and expansion of the mitochondrial (ATP + ADP) pool contribute to postnatal maturation of the rat heart. Eur J Biochem. 1996;241:895-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Dolce V, Scarcia P, Iacopetta D, Palmieri F. A fourth ADP/ATP carrier isoform in man: identification, bacterial expression, functional characterization and tissue distribution. FEBS Lett. 2005;579:633-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 180] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Chen LF, Liu JZ, Song R, Dang YM. Roles of CAP-treatment for respiratory function of mitochondria from cerebral cortex of acute hypoxic exposure rats. Disan Junyi Daxue Xuebao. 2004;26:1611-1614. |
| 4. | Gao WX, Liu JZ, Wu LP, Cai CM. Characteristics of energy metabolism in brain mitochondria of rats exposured to hypoxia. Zhongguo Bingli Shenglixue Zazhi. 2000;16:879-882. |
| 5. | Cardoso CM, Moreno AJ, Almeida LM, Custódio JB. Comparison of the changes in adenine nucleotides of rat liver mitochondria induced by tamoxifen and 4-hydroxytamoxifen. Toxicol In Vitro. 2003;17:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Li B, Liu JZ, Chen FL. Changes of adenylate content and distribution in myocardium and mitochondria of rats after hypoxic exposure. Xibei Guofang Yixue Zazhi. 2005;26:90-92. |
| 7. | Liu JZ, Gao WX, Cao LF, Cai MC. changes of adenylate pool and energy change in mitochondria isolate from rat brain exposued to hypobaric hypoxia. Disan Junyi Daxue Xuebao. 2003;25:2165-2168. |
| 8. | Garboczi DN, Hullihen JH, Pedersen PL. Mitochondrial ATP synthase. Overexpression in Escherichia coli of a rat liver beta subunit peptide and its interaction with adenine nucleotides. J Biol Chem. 1988;263:15694-15698. [PubMed] |
| 9. | Haynes RC Jr, Picking RA, Zaks WJ. Control of mitochondrial content of adenine nucleotides by submicromolar calcium concentrations and its relationship to hormonal effects. J Biol Chem. 1986;261:16121-16125. [PubMed] |
| 10. | Austin J, Aprille JR. Carboxyatractyloside-insensitive influx and efflux of adenine nucleotides in rat liver mitochondria. J Biol Chem. 1984;259:154-160. [PubMed] |
| 11. | Thomson L, Gadelha FR, Peluffo G, Vercesi AE, Radi R. Peroxynitrite affects Ca2+ transport in Trypanosoma cruzi. Mol Biochem Parasitol. 1999;98:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Belzacq AS, Vieira HL, Kroemer G, Brenner C. The adenine nucleotide translocator in apoptosis. Biochimie. 2002;84:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Rulfs J, Aprille JR. Adenine nucleotide pool size, adenine nucleotide translocase activity, and respiratory activity in newborn rabbit liver mitochondria. Biochim Biophys Acta. 1982;681:300-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Krämer R, Klingenberg M. Modulation of the reconstituted adenine nucleotide exchange by membrane potential. Biochemistry. 1980;19:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Passarella S, Ostuni A, Atlante A, Quagliariello E. Increase in the ADP/ATP exchange in rat liver mitochondria irradiated in vitro by helium-neon laser. Biochem Biophys Res Commun. 1988;156:978-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 90] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, Trézéguet V, Lauquin GJ, Brandolin G. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature. 2003;426:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 771] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 17. | Pebay-Peyroula E, Brandolin G. Nucleotide exchange in mitochondria: insight at a molecular level. Curr Opin Struct Biol. 2004;14:420-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |