Published online Apr 7, 2006. doi: 10.3748/wjg.v12.i13.2060
Revised: November 11, 2005
Accepted: November 18, 2005
Published online: April 7, 2006
AIM: Most gastrointestinal stromal tumors (GISTs) express constitutively activated mutant isoforms of kit kinase or platelet-derived growth factor receptor alpha (PDGFRA), which are potential therapeutic targets for imatinib mesylate (Glivec). Partial response occurred in almost two thirds of GIST patients treated with Glivec. However, complete response (CR) after Glivec therapy was sporadically reported. Here we illustrated advanced GIST patients with CR after Glivec treatment.
METHODS: Between January 2001 and June 2005, 42 advanced GIST patients were treated with Glivec. Patients were administered 400 mg of Glivec in 100-mg capsules, taken orally daily with food. The response of the tumor to Glivec was evaluated after one month, three months, and every three months thereafter or whenever medical need was indicated. Each tumor of patients was investigated for mutations of kit or PDGFRA.
RESULTS: The median follow-up time of the 42 ad-vanced GIST patients treated with Glivec was 16.9 months (range, 1.0 - 47.0 months). Overall, 3 patients had complete response CR (7.1 %), 26 partial response (67.8 %), 5 stationary disease (11.9 %), and 3 progressive disease (11.9 %). The median duration of Glivec administration for the three patients was 36 months (range, 23-36 months). The median time to CR after Glivec treatment was 20 months (range, 9-26 months). Deletion and insertion mutations of c-kit exon 11 and insertion mutation of c-kit exon 9 were found in two cases and one case, respectively.
CONCLUSION: Complete response (CR) can be achi-eved in selected advanced GIST patients treated with Glivec. The median time to CR after Glivec treatment was 20 months. Deletion and insertion mutations of kit exon 11 and insertion mutation of kit exon 9 contribute to the genetic features in these selected cases.
- Citation: Chiang KC, Chen TW, Yeh CN, Liu FY, Lee HL, Jan YY. Advanced gastrointestinal stromal tumor patients with complete response after treatment with imatinib mesylate. World J Gastroenterol 2006; 12(13): 2060-2064
- URL: https://www.wjgnet.com/1007-9327/full/v12/i13/2060.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i13.2060
Gastrointestinal stromal tumors (GISTs) are soft-tissue sarcomas primarily arising from mesenchymal tissue in the gastrointestinal (GI) tract and abdomen. They are rare neoplasms, estimated to represent 0.1 to 3 % of all GI tract tumors[1]. However, GISTs are the most common mesenchymal malignancy of the GI tract with precise incidence unknown[2]. GISTs appear to be related to the interstitial cells of Cajal of the mesenteric plexus[3]. These cells are considered GI pacemaker cells, from the interface between the automatic innervation of the bowel wall and its smooth muscle[4,5]. GISTs express the cell-surface transmembrane receptor kit with a tyrosin kinase activity and is the protein product of the kit proto-oncogene. There are frequent gain-of-function mutations of kit in GISTs. These mutations result in constitutive activation of kit signaling, which leads to uncontrolled cell proliferation and resistance to apoptosis. It has been recently reported that kit activation occurs in all cases of GISTs, regardless of the mutation status of kit.
Surgical resection remains the mainstay of therapy for GIST. However, recurrence is common; the 5-year survival rates after complete resection range from 40 to 65 %[6-10]. Unresectable or metastatic GIST is a fatal disease that resists conventional chemotherapy. In a recently reported series, the response rate to doxorubicin therapy was less than 5 %[11]. The effectiveness of radiation therapy for unresectable or metastatic GIST has not been proved. The median length of survival for patients with a metastatic GIST is approximately 20 months, and 9 to 12 months for patients with local recurrence[3]. Before the development of Glivec, the outlook for patients with advanced GIST was extremely poor. A significantly large number of patients with initial resection of GIST eventually experience recurrence, for which there is no effective treatment.
Imatinib mesylate (formerly STI571, now referred to as Gleevec in the United States and Glivec in Europe [Novartis]) selectively inhibits certain protein tyrosin kinases: intracellular ABL kinase, chimeric BCR-ABL fusion oncoprotein of chronic myeloid leukemia, transmembrane receptor kit, and platelet-derived growth factor (PDRGF) receptors[12-15]. Glivec induced a sustained objective response in more than half of patients with advanced GISTs[16]. However, complete response (CR) induced by Glivec on GIST patients has been sporadically reported. We report herein our experience on three GIST patient treated with Glivec achieving complete response.
During January 2001 to May 2005, 42 histologically con-firmed, unresectable or metastatic GIST patients ex-pre-ssing CD117 (a marker of kit-receptor tyrosine kinase) and CD34 treated at Department of Surgery, Chang Gung Memorial Hospital, Taiwan were enrolled in this study. Metastatic disease was defined as that occurring at structures noncontiguous with the primary tumor site. Criteria for inclusion were as follows: at least one measur-able tumor; adequate hepatic, renal, and cardiac function; an adequate platelet count; and an Eastern Coo-perative Oncology Group (ECOG) performance status of 3 or less. Patients could have previously received che-motherapeutic regimens (the last chemotherapy treatment must have been at least four weeks before the study entry) and undergone radiotherapy, or surgery, or both. R0 resection means curative resection without microscopic evidence of tu-mor. R2 resection means resection with macroscopic evidence of tumor. The study was approved by the Local Institutional Review Board of Chang Gung Memorial Hospital and written informed consent for drug administration and the analysis of tumor-associated genetic alteration was obtained from each patient.
A prospective, non-randomized, and single center trial was conducted to evaluate the role of Glivec in inducing objec-tive response in GIST patients. Patients were administered 400 mg of Glivec in 100-mg capsules, taken orally daily with food. Patients had regular physical examinations and evaluations of performance status, body weight, complete blood count, and serum chemistry. The administration of each dose and any adverse events were recorded for each patient. Standard computed tomography (CT) was performed on each patient every three months to assess patient response. Standard [18F] fluoro-2-deoxy-D-glucose positron-emission tomography (PET) scanning was performed on selected patients to complement standard CT and assess changes in the metabolic profiles of the tumors.
The response of the tumor to Glivec was evaluated aft-er one month, three months, and every three months thereafter or whenever medical need was indicated. Ass-essments were performed according to the standard Sou-thwest Oncology Group (SWOG) criteria and based solely on CT or PET[17]. Responses were classified as follows: complete response (CR) (disappearance of all disease that could be measured and evaluated); partial response (PR) (> 50 % decrease in the sum of the products of the perpendicular diameters of all measurable lesions, the absence of progression, and the absence of new lesions);stationary disease (SD) (a response that did not qualify as a complete response, a partial response, or disease pro-gression); and disease progression (DP) [> 50 % increase or an increase of 10 cm (whichever was smaller) in the sum of the products of the perpendicular diameter of all measurable lesions, worsening of a lesion that could be evaluated, the reappearance of any lesion or the presence of a new lesion, or failure of the patients to return for evaluation because of disease progression]. Toxic effects were recorded in accordance with the National Cancer Institute Common Toxicity Criteria[18].
Sections were prepared from formalin-fixed, paraffin-embedded pretreatment specimens trimmed to enrich tumor cells. Polymerase chain reaction amplification of genomic DNA for KIT and PDGFRA was performed and amplification was analyzed for mutations as previously described[19].
The investigation comprised one male patient and two female patients with ages ranging from 45 to 56 years (median: 51 years) (Table 1). All three patients had grade 0 ECOG status.
| Patient | 1 | 2 | 3 |
| Age (yr) | 57 | 45 | 51 |
| Gender | F | M | F |
| ECOG | Grade 0 | Grade 0 | Grade 0 |
| Tumor origin | Jejunum | Stomach | Jejunum |
| Tumor size (cm) | 20 | 10 | 10 |
| Previous treatment | Operation | Laparotomy and excisional biopsy | Operation |
| Resection | R0 | R2 | R0 |
| Site of tumor recurrence | Liver, locoregional, and peritoneum | Liver, peritoneum, and retroperitoneum | Peritoneum |
| Interval between previous treatment and recurrence (mo) | 15 | 0 | 18 |
| Glivec dose/duration (mo) | 400/36 | 400/23 | 400/36 |
| Side effect Mutation status | Grade II edema Deletion and insertion mutation at codons 563-572 in exon 11 | Grade III edema Deletion and insertion mutation at codons 556-557 in exon 11 | Grade II edema Insertion AY at codons 502-503 in exon 9 |
| Time to CR (mo) | 20 | 9 | 26 |
| CT | CR | CR | CR |
| PET | CR without activity | CR without activity | CR without activity |
| Duration of response (mo) | 16 | 14 | 10 |
| Overall survival (mo) | 40 | 24 | 54 |
| Status | Free of disease | Free of disease | Free of disease |
Table 1 summarizes the size and location of each tumor.One patient underwent laparotomy with excisional biopsy and the other two had curative segmental resection of jejunal GIST previously. Tumors of all three patients di-splayed strong positive kit staining with the tumor size ranging from 10 cm to 20 cm (median: 10 cm). The inter-val between diagnosis of GIST and tumor recurrence ranged from 0 to 15 months (median 7 months). All three patients displayed peritoneal carcinomatosis and two had liver metastasis.
All three patients were administered 400 mg Glivec after diagnosis of metastasis was made. The duration of Glivec administration ranged from 24 to 36 months (median: 36 months). The side effect of Givec treatment was grade II to III edema.
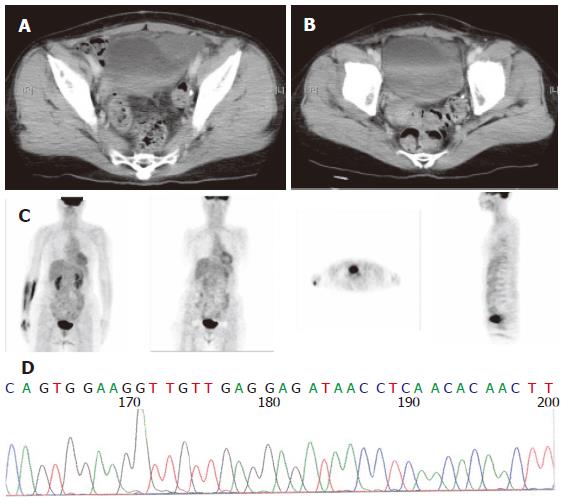
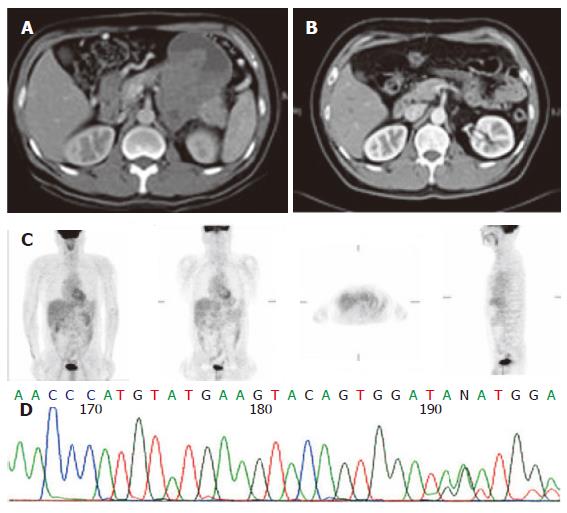
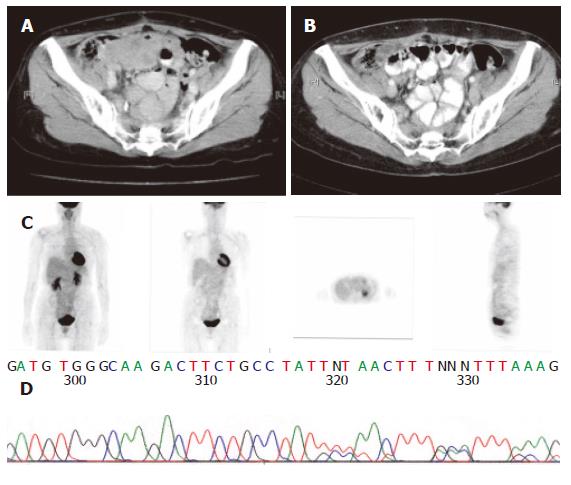
The sequencing analysis of the tumor from the three pa-tients exhibited mutation in c-kit gene. Two displayed deletion and insertion mutation in exon 11 and one inser-tion mutation in exon 9 (Figure 1, Figure 2 and Figure 3).
Since 2000, Glivec has been administered to advanced GIST patients. Forty-two patients with advanced stages of the disease were given 400 mg Glivec per day. The median follow-up duration was 16.9 months (range, 1.0-47.0 months). Overall, 3 (7.1 %) patients had complete response (CR), 26 (67.8 %) partial response, 5 (11.9 %) stationary disease, 3 progressive disease, and 3 (7.2 %) patients were unavailable to evaluate. The time to CR after Glivec treatment ranged from 9 to 26 months (median, 20 months) as illustrated by CT first and confirmed by PET without any metabolic activity (Figures 1, 2 and 3). The median follow-up period of the three advanced GIST patients treated with Glivec with CR was 40 months (range, 24 - 54 months).
Before the introduction of Glivec, poor responses to radiation and chemotherapy made surgery the only realistic treatment to cure the primary lesion[3,10,20-22]. A substantial number of patients with initial resection of GISTs even-tually experience recurrence. There has been no effective treatment for advanced GISTs and the outlook for patients is extremely poor.
Therapeutic responses to targeted inhibition of activat-ed tyrosine kinases have been demonstrated for certain types of leukemia, sarcoma, and breast cancer[19]. The mechanisms of kinase activation vary considerably among these cancers, but there is little information available in literature about the influence of these mechanisms on dr-ug response[19]. The GISTs, in particular, present a variety of genomic mutations across two different receptor tyro-sine kinase genes. The KIT or PDGFRA mutation in Asian clinically advanced small bowel GIST patients was examined in this study. The kit kinase oncoproteins were intrinsically sensitive to Glivec, accounting for the excellent overall clinical response to Glivec, and coincident with results obtained by Heinrich et al[19]. Similar to the report by Demetri’s et al[16], the CR and PR rates for Glivec in this study was 68.0 %. Glivec induced a sustained objective response in more than half of the patients with advanced GISTs[16]. However, CR induced by Glivec on GIST patients was sporadically reported. In US Intergroup S0033 phase III study on 751 metastatic or unresectable GIST patients receiving 400 or 800 mg Glive per day[23], CR rate was 3 %. Moreover, in the EORTC 62005 phase III study, the CR rate was 4.76 % for 923 metastatic or unresectable receiving 400 or 800 mg Glive per day. Contrast to the aforementioned two studies, the CR rate in this study was 7 %. The experience on CR after Glivec treatment for advanced or metastatic GIST patients in this study may justify the use of Glivec as neoadjuvant or adjuvant treatment in the future. FDG PET has been proven to be highly sensitive in detecting early response[24]. Stroobants et al[24] demonstrated that the CR rate increased to 52.3 % (11/21), however, discrepancy was noted between the CT and PET results. In this study, CR was diagnosed according to SWOG criteria by CT scan first. PET scan was used to confirm its metabolic activity by FDG uptake on PET scan thereafter.
Regarding further use of Glivec for GIST patients with CR after Glivec treatment, no consensus was made. A recently reported randomized trial has shown that Glivec interruption after 1 year is associated with a high risk of relapse, even for patients with CR[25]. So, Glivec might be administered in the three patients until intolerance or patient refusal. The further use of Glivec for GIST patients with CR after Glivec treatment needs investigation.
In conclusion, CR can be achieved in selected patients with advanced GIST treated with Glivec. Deletion and insertion mutations of kit exon 11 and insertion mutation of kit exon 9 contribute to the genetic features in these selected cases.
We thank Novartis (Taiwan) Co., Ltd for financial support of genetic analysis and PET scan.
S- Editor Wang J L- Editor Kumar M E- Editor Wu M
| 1. | Lewis JJ, Brennan MF. Soft tissue sarcomas. Curr Probl Surg. 1996;33:817-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 146] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Rossi CR, Mocellin S, Mencarelli R, Foletto M, Pilati P, Nitti D, Lise M. Gastrointestinal stromal tumors: from a surgical to a molecular approach. Int J Cancer. 2003;107:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Rossi CR, Mocellin S, Mencarelli R, Foletto M, Pilati P, Nitti D, Lise M. Gastrointestinal stromal tumors: from a surgical to a molecular approach. Int J Cancer. 2003;107:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259-1269. [PubMed] |
| 5. | Sircar K, Hewlett BR, Huizinga JD, Chorneyko K, Berezin I, Riddell RH. Interstitial cells of Cajal as precursors of gastrointestinal stromal tumors. Am J Surg Pathol. 1999;23:377-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 337] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 6. | Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1430] [Cited by in RCA: 1326] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 7. | Akwari OE, Dozois RR, Weiland LH, Beahrs OH. Leiomyosarcoma of the small and large bowel. Cancer. 1978;42:1375-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Shiu MH, Farr GH, Papachristou DN, Hajdu SI. Myosarcomas of the stomach: natural history, prognostic factors and management. Cancer. 1982;49:177-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | McGrath PC, Neifeld JP, Lawrence W, Kay S, Horsley JS, Parker GA. Gastrointestinal sarcomas. Analysis of prognostic factors. Ann Surg. 1987;206:706-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Ng EH, Pollock RE, Munsell MF, Atkinson EN, Romsdahl MM. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg. 1992;215:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 286] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Goss GA, Merriam P, Manola . Clinical and pathological characteristics of gastrointestinal stromal tumors (GIST). Prog Proc Am Soc Clin Oncol. 2000;19:559a. |
| 12. | Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2692] [Cited by in RCA: 2582] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 13. | Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, Lydon NB. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000;295:139-145. [PubMed] |
| 14. | Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000;96:925-932. [PubMed] |
| 15. | Wang WL, Healy ME, Sattler M, Verma S, Lin J, Maulik G, Stiles CD, Griffin JD, Johnson BE, Salgia R. Growth inhibition and modulation of kinase pathways of small cell lung cancer cell lines by the novel tyrosine kinase inhibitor STI 571. Oncogene. 2000;19:3521-3528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 177] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3203] [Cited by in RCA: 3110] [Article Influence: 135.2] [Reference Citation Analysis (0)] |
| 17. | Green S, Weiss GR. Southwest Oncology Group standard response criteria, endpoint definitions and toxicity criteria. Invest New Drugs. 1992;10:239-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 470] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 18. | Cancer Therapy Evaluation Program. Common toxicity criteria manual: common toxicity criteria, version 2.0. Bethesda, MD: National Cancer Institute 1999; Jun. |
| 19. | Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342-4349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1817] [Cited by in RCA: 1646] [Article Influence: 74.8] [Reference Citation Analysis (1)] |
| 20. | Langer C, Gunawan B, Schüler P, Huber W, Füzesi L, Becker H. Prognostic factors influencing surgical management and outcome of gastrointestinal stromal tumours. Br J Surg. 2003;90:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Pierie JP, Choudry U, Muzikansky A, Yeap BY, Souba WW, Ott MJ. The effect of surgery and grade on outcome of gastrointestinal stromal tumors. Arch Surg. 2001;136:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 208] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Plaat BE, Hollema H, Molenaar WM, Torn Broers GH, Pijpe J, Mastik MF, Hoekstra HJ, van den Berg E, Scheper RJ, van der Graaf WT. Soft tissue leiomyosarcomas and malignant gastrointestinal stromal tumors: differences in clinical outcome and expression of multidrug resistance proteins. J Clin Oncol. 2000;18:3211-3220. [PubMed] |
| 23. | Rankin C, von Mehren M, Blanke C. Continued prologation of survival by imatinib in patients with metastatic GIST. Update of results from North American Intergroup phase III study S0033. Proc Am Soc Clin Oncol. 2004;23:815. |
| 24. | Stroobants S, Goeminne J, Seegers M, Dimitrijevic S, Dupont P, Nuyts J, Martens M, van den Borne B, Cole P, Sciot R. 18FDG-Positron emission tomography for the early prediction of response in advanced soft tissue sarcoma treated with imatinib mesylate (Glivec). Eur J Cancer. 2003;39:2012-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 352] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 25. | Blay JY, Berhaud P, Perol D. Continous vs intermittent imatinib treatment in advanced GIST after one year: A prospective randomized phase III trial of the French Sarcoma Group. Proc Am Soc Clin Oncol. 2004;23:815. |