Published online Feb 21, 2005. doi: 10.3748/wjg.v11.i7.982
Revised: June 27, 2004
Accepted: July 27, 2004
Published online: February 21, 2005
AIM: To investigate the effectiveness of 4 d’ anti-Helicobacter pylori therapy on the H pylori-infected Mongolian gerbils based on physiological and pathological changes.
METHODS: We used 6-wk-old male gerbils orally inoculated with H pylori (ATCC43504, 2x108 CFU/mL). Seven weeks after H pylori inoculation, the animals of study group received 4 d’ anti-H pylori triple therapy (H pylori-eradicated group). Seven days later, all animals of the H pylori-eradicated and control groups (H pylori-infected & H pylori-uninfected groups) were sacrificed. We examined gastric mucosal lesions macroscopically, studied gastritis microscopically and determined the stomach weight ratio, myeloperoxidase (MPO) activity and prostaglandin (PG) E2 level.
RESULTS: The results showed that both macroscopic and histological gastric damages were significantly less in H pylori-eradicated group than H pylori-infected group. Stomach weight ratio, MPO activity and PGE2 levels were significantly higher in H pylori-infected group than those in the other two groups.
CONCLUSION: Four days’ anti-H pylori therapy was effective in the improvement of H pylori-induced gastric lesions in Mongolian gerbils.
-
Citation: Chang CC, Chen SH, Lien GS, Lee YJ, Lou HY, Hsieh CR, Fang CL, Pan S. Anti-
Helicobacter pylori therapy significantly reducesHelicobacter pylori -induced gastric mucosal damage in Mongolian gerbils. World J Gastroenterol 2005; 11(7): 982-985 - URL: https://www.wjgnet.com/1007-9327/full/v11/i7/982.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i7.982
Helicobacter pylori (H pylori) is considered a major cause of acute and chronic gastritis as well as peptic ulcer disease, and is also highly associated with the development of gastric mucosa-associated lymphoid tissue lymphoma and gastric cancer[1-3]. Both National Institute of Health (NIH) of USA and International Agency for Research on Cancer (IARC) have issued statements on the importance of H pylori eradication and carcinogenic risks in patients with H pylori infection[4,5]. At present, H pylori eradication therapy is performed in patients with peptic ulcer diseases. In 1996, Hirayama et al[6] reported that H pylori infection in Mongolian gerbils can induce gastric ulcer and intestinal metaplasia. This animal model is considered to be very similar to human H pylori infection. However, little progress has been made in the H pylori-eradication with a simple regimen and a short-term course. Therefore, we conducted this study to compare histological and physiological changes in the stomach of Mongolian gerbils including those receiving 4 d’ anti-H pylori therapy after a successful inoculation of H pylori, with those infected with H pylori but not receiving anti-H pylori therapy, and those not infected with H pylori.
Six-week-old specific pathogen-free male Mongolian gerbils (MGS/Sea) weighing 40-50 g, were used. The animals were kept in an isolated clean room with regulated temperature (approximately 20-22 °C) and humidity (approximately 55%) with a 12/12-h light-dark cycle. The animals were allowed free access to water and sterile solid food (CE-2). There were 3 groups of experimental animals prepared for this study: group A, not inoculated with H pylori (H pylori-uninfected group, n = 7); group B, inoculated with H pylori but not received anti-H pylori therapy (H pylori-infected group, n = 7); and group C, inoculated with H pylori, and treated with anti-H pylori triple therapy for 4 d (H pylori-eradicated group, n = 7).
ATCC43504, a standard H pylori strain (CagA and VacA-positive), was used. The bacteria were incubated overnight in a brain-heart infusion broth (Difco Laboratories, Detroit, Michigan) containing 10% fetal bovine serum (Gibco BRL, Grand Island, New York) at 37 °C under a microaerophilic atmosphere (N2 85%, CO2 10%, O2 5%), and allowed to grow to a concentration of approximately 2.0×108 colony-forming units (CFU)/mL.
The animals were deprived of food for 24 h before and 4 h after H pylori inoculation, but were otherwise afforded free access to food and tap water. One mL of H pylori (2.0×108 CFU/mL) was orally inoculated into animals of groups B and C.
The regimen of anti-H pylori triple therapy is same as the therapy used in human beings. The drugs including lansoprazole, amoxicillin and clarithromycin were suspended in 0.5% w/w carboxymethyl cellulose (CMC) sodium salt solution and administrated orally twice a day for four days at a dose of 10, 3, and 30 mg/kg body weight respectively.
The animals of group C received 4 d’ anti-H pylori therapy at 7 wk after H pylori inoculation, while the animals of other 2 groups (groups B and C) received vehicle (CMC) for 4 d.
All animals were sacrificed 7 d after cessation of medication.
We performed H pylori culture and rapid urease test to confirm the existence of H pylori.
The gerbils were sacrificed with ether anesthesia, and then their half-stomachs (right side) were excised. After approximate 50-100 g of the stomach was punched out for MPO activity examination, the remaining part of the stomach was homogenized in 10 mL phosphate buffered saline with a Polytron, followed by dilution with the same buffer. Aliquots (100 μL) of the dilutions were applied to Brucella agar plates containing 10% horse blood (Nippon Bio-Test Laboratories, Tokyo, Japan), 2.5 μg/mL amphotericin B, 9 μg/mL vancomycin, 0.32 μg/mL polymyxin B, 5 μg/mL trimethoprim and 50 μg/mL 2,3,5-triphenyltetrazolium chloride. The plates were incubated at 37 °C under microaerophilic atmosphere (N2 850 mL/L, CO2 100 mL/L, O2 5%) for seven days. H pylori was identified as gold colonies in spiral shape under a microscope and positive for rapid urease test.
Gross observation The stomachs were opened along the greater curvature and washed with phosphate-buffered saline, then were spread gently and fixed with pins on a cork board. All the stomachs were observed by a researcher who was unaware of the treatment of the animals. Under dissecting microscope, variable size and type of gastric lesions were checked and recorded with a square grid.
Microscopic observation Half of each stomach (left side) was fixed in 10% formalin and embedded with paraffin. Four μm thick sections were prepared and stained with hematoxylin and eosin. The diagnosis of gastritis was made according to the modified criteria, which was slightly modified from Rauws et al[7]. The parameters of chronic active gastritis were as follows: lymphocyte infiltration (0: none, 1: mild infiltration to lamina propria, 2: moderate infiltration to lamina propria, 3: severe infiltration and lymphoid follicle formation), polymorphonuclear leukocyte infiltration (0: none, 1: the number of cells in lamina propria <30 in the field of magnification ×400, 2: 30-100/field, 3: >100/field), superficial erosions (0: none, 1: deletion of surface epithelial cells). The total score of these variables varied from 0 to 7, and was used as a measure of the activity of gastritis. Microscopic gastritis was classified as non-gastritis (score: 0), mild gastritis (score: 1-3), moderate gastritis (score: 4-5) and severe gastritis (score: 6-7). An experienced pathologist determined the gastric pathology without himself being aware of the prior treatment.
PGE2 production in the gastric mucosa of the gerbils was determined according to the method of Lee and Feldman[8]. We punched out about 50-100 mg gastric specimen from the border of gastric antrum and corpus (right half side) in each gerbil. The specimen was placed in 50 mmol/L Tris HCl (pH 8.4) buffer and then minced with a pair of scissors. After the tissue samples were washed and resuspended in 2 mL of buffer, each sample was subjected to vortex mixing at room temperature for 1 min to stimulate PGE2 production, followed by centrifugation at 10000 g for 15 s. The PGE2 levels in the resulting supernatants were determined by means of enzyme immunoassay (PGE2 EIA kit; Cayman Chemicals, Ann Arbor, Michigan, USA). PGE2 production was expressed as picograms of PGE2 per minute per milligram of tissue.
After removal of supernatants for PGE2 analysis, the MPO activity in the remaining tissue was determined with the method described by Takahashi and Keto[9]. Each sample (approximately 50-100 mg) was first homogenized with a Polytron in 1.0 mL of 50 mmol/L phosphate buffer (pH 6.0) containing 0.5% hexadecyltrimethylammonium bromide(Sigma), and then subjected to three sessions of freezing-thawing. Subsequently, the homogenates were centrifuged at 1600 g for 10 min at 4 °C. After 5 μL aliquot of each supernatant was mixed with 145 μL of phosphate buffer (pH 6.0) containing 0.167 mg/mL o-dianisidine dihydrochloride (Sigama) and 0.0005% H2O2, the change in the rate of absorbance at 450 nm was measured with microplate reader (Thermo Max; Molecular Devices, Sunnyvale, CA). The MPO activity was expressed as the degradation of H2O2μmoL/min/g tissue.
The data was presented as mean±SE and were analyzed with Student’s t-test. P value less than 0.05 was regarded as statistically significant.
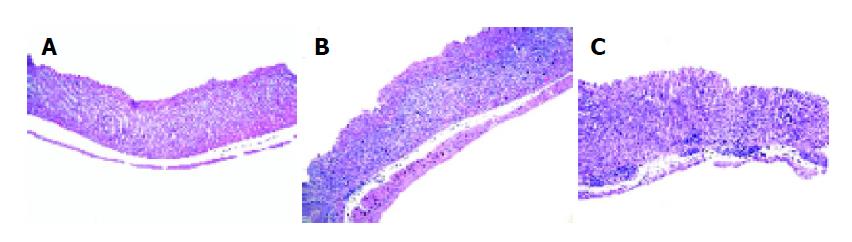
Among the H pylori-eradicated animals, 6 out of 7 gerbils were free of H pylori infection. The eradication rate was around 85.7%. The mean body weights of animals in groups A, B and C were 76.3±3.4, 73.0±1.6 and 76.1±2.5 g respectively. The difference of mean body weight in the 3 groups was not statistically significant (P>0.05). There was no gastritis in group A (Figure 1A). Mild to moderate gastritis including erosions and hyperplasia of mucosa were found in group C (Figure 1B). However, remarkable erosions and hyperplasia of mucosa were found in group B (Figure 1C). The mean area of erosions (mm2) of gastric mucosa in groups A, B and C were 0±0, 2.2±0.6, 0.4±0.3 respectively. The differences in area of gastric erosions between group A and group B and between group B and group C were statistically significant (P<0.05) (Table 1). The mean area (mm2) of hyperplasia of gastric mucosa in groups A, B and C were 0±0, 62.8±6.3, 0.9±0.6 respectively. The differences in area of hyperplasia of gastric mucosa between group A and group B and between group B and group C were statistically significant (P<0.05) (Table 1). The mean scores of gastritis by histological study in groups A, B and C were 0±0, 6.9±0.1, 2.0±0.5 respectively. The difference was statistically significant between two groups (group A vs group B, P<0.001; group B vs group C, P<0.001) (Table 1).
| Hp-infected Group A (n: 7) | Hp-eradicated Group B (n: 6) | Hp-uninfected Group C (n: 7) | P value | |
| Stomach weight ratio (×1000) | 14.1±0.3 | 9.8±0.2 | 8.9±0.3 | <0.05a |
| <0.05c | ||||
| Hyperplasia area (mm2) | 62.8±6.3 | 0.9±0.6 | 0 | <0.05a |
| <0.05c | ||||
| Erosion and ulcer area (mm2) | 2.2±0.6 | 0.4±0.3 | 0 | <0.05a |
| <0.05c | ||||
| Score of gastritis | 6.9±0.1 | 2.0±0.5 | 0 | <0.05a |
| <0.05c | ||||
| MPO activity (mmol H2O2/min/g tissue) | 100.4±5.9 | 5.7±1.3 | 4.6±2.3 | <0.05a |
| <0.05c | ||||
| PGE2 synthesis (pg/mg wet weight/min) | 77.0±15.3 | 30.6±11.9 | 26.9±14.4 | <0.05a |
| <0.05c |
In addition, the weight of stomach increased remarkably after H pylori infection. The mean wet weights (gram) of the stomach in groups A, B and C were 8.9±0.3, 14.1±0.3, 9.8±0.2 respectively. The differences between groups A and B (P<0.001) and groups B and C (P<0.001) were of statistical significance (Table 1).
The values of MPO activity (μmol H2O2/min/g tissue) in groups A, B and C were 4.6±2.3, 100.4±5.9, 5.7±1.3 respectively. The differences between groups A and B (P<0.001) and between groups B and C (P<0.001) were statistically significant (Table 1).
The values of PGE2 synthesis (pg/mg wet weight/min) in groups A, B and C were 26.9±14.4, 77.0±15.3, 30.6±11.9 respectively. The differences between groups A and B (P<0.001) and between groups B and C (P<0.001) were statistically significant (Table 1).
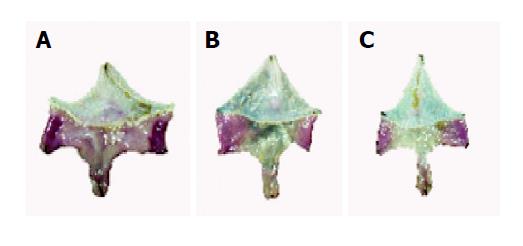
Although we did not measure the thickness of the stomach, it became more thicker in H pylori-infected gerbils than H pylori-uninfected or H pylori-eradicated gerbils (Figure 2A-C).
Hirayama et al[6,10] has demonstrated gastritis, gastric erosions, gastric ulcer and intestinal metaplasia from the stomach of Mongolian gerbils infected with H pylori in different periods. Sawada et al[11] found acute inflammation, immature epithelium and erosion can be observed 2 wk after H pylori infection, while chronic inflammation can be noted 4 wk after H pylori infection. In addition, intestinal metaplasia and gastric ulcers develop 12 and 24 wk after H pylori infection respectively. Takahashi et al[9] study found edematous and/or congestive mucosa and white substance in the stomachs from Mongolian gerbils 1-4 wk after infection with H pylori. However, there is no report about the efficient and rapid eradication of H pylori in Mongolian gerbils. Keto et al[12] concluded that combined treatment with omeprazole and clarithromycin for 4 wk can enhance healing of H pylori-induced gastric ulcers in Mongolian gerbils, but it took a long period of time to eradicate H pylori in their experiment. In addition, Kusuhara et al[13] eradicated H pylori in Mongolian gerbils 5 d after dual therapy (amoxicillin plus omeprazole or clarithromycin plus omeprazole). However, dual therapy for H pylori eradication in human beings is used less frequently. In our study, we used anti-H pylori triple therapy, which is commonly used in the treatment for humans infected with H pylori. The drugs we used were amoxicillin, clarithromycin and lansoprazole. The eradication rate was 85.7% in our study.
From gross observation, there were less superficial lesions (erosions and hyperplasia) in H pylori-eradicated gerbils than in H pylori-infected gerbils. It revealed that effective eradication of H pylori can reduce the gastric inflammation in Mongolian gerbils. Probably, the interval between stopping use of antibiotics and sacrifice of animals was too short, so that gastric inflammation in H pylori-eradicated animals did not heal completely. The scores of gastritis in H pylori-uninfected and H pylori-eradicated animals were less than that in H pylori-infected animals.
From our study, we observed that H pylori eradication can effectively reduce gastric inflammation including erosion and hyperplasia of gastric mucosa in Mongolian gerbils. Anti-H pylori triple therapy can be applied in the study of Mongolian gerbils infected with H pylori for one to two years. Probably, it is helpful in the investigation - can the eradication of H pylori decrease adenomas and/or hyperplastic polyps in Mongolian gerbils?
There are many risk factors involved for development of gastritis including H pylori, non-steroidal anti-inflammatory drugs (NSAIDs), cigarette smoking, ethanol, food, etc. Recently, Suzuki et al[14] reported that ethanol intake preceding H pylori inoculation promotes gastric mucosal inflammation in Mongolian gerbils. Takahashi et al[15] reported that NSAIDs induce acute gastric injury in Mongolian gerbils. Tanigawa et al[16] suggested that fish meal contains factors, which greatly enhance H pylori-induced gastritis in Mongolian gerbils. Perhaps, we can investigate the effects of eradicating H pylori prior to administration of ethanol, NSAIDs and fish meal in Mongolian gerbils. It is very important to elucidate the necessity of eradicating H pylori in patients who use NSAIDs and those who are heavy drinkers.
In conclusion, 4 d’ anti-H pylori therapy is a rapid and effective modality in eradicating H pylori in Mongolian gerbils and it can be used in the study of gastric mucosal changes in gerbils infected with H pylori.
We are grateful to Professor Susumu Okabe and Kikuko Amagase for providing valuable instructions in conducting this study. This study was supported by the grant from TMU-Y05-A108.
| 1. | Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273-1275. [PubMed] |
| 2. | Hentschel E, Brandstätter G, Dragosics B, Hirschl AM, Nemec H, Schütze K, Taufer M, Wurzer H. Effect of ranitidine and amoxicillin plus metronidazole on the eradication of Helicobacter pylori and the recurrence of duodenal ulcer. N Engl J Med. 1993;328:308-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 465] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 3. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2805] [Cited by in RCA: 2739] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 4. | NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA. 1994;272:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 752] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 5. | IARC Monographs on the evaluation of carcinogenic risks to human. Schistomomes, liver flukes and Helicobacter pylori: Geneva: WHO, International agency for research on cancer. Monography. 1994;66:177-220. |
| 6. | Hirayama F, Takagi S, Kusuhara H, Iwao E, Yokoyama Y, Ikeda Y. Induction of gastric ulcer and intestinal metaplasia in mongolian gerbils infected with Helicobacter pylori. J Gastroenterol. 1996;31:755-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 148] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Rauws EA, Langenberg W, Houthoff HJ, Zanen HC, Tytgat GN. Campylobacter pyloridis-associated chronic active antral gastritis. A prospective study of its prevalence and the effects of antibacterial and antiulcer treatment. Gastroenterology. 1988;94:33-40. [PubMed] |
| 8. | Lee M, Feldman M. Age-related reductions in gastric mucosal prostaglandin levels increase susceptibility to aspirin-induced injury in rats. Gastroenterology. 1994;107:1746-1750. [PubMed] |
| 9. | Takahashi S, Keto Y, Fujita H, Muramatsu H, Nishino T, Okabe S. Pathological changes in the formation of Helicobacter pylori-induced gastric lesions in Mongolian gerbils. Dig Dis Sci. 1998;43:754-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Hirayama F, Takagi S, Yokoyama Y, Iwao E, Ikeda Y. Establishment of gastric Helicobacter pylori infection in Mongolian gerbils. J Gastroenterol. 1996;31 Suppl 9:24-28. [PubMed] |
| 11. | Sawada Y, Sashio H, Yamamoto N, Hida N, Akashi H, Tonokatsu Y, Sakagami T, Fukuda Y, Shimoyama T, Nishigami T. Pathologic changes in the glandular stomach and duodenum in an H. pylori-infected Mongolian gerbil model. J Clin Gastroenterol. 1998;27 Suppl 1:S141-S143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Keto Y, Takahashi S, Okabe S. Healing of Helicobacter pylori-induced gastric ulcers in Mongolian gerbils: combined treatment with omeprazole and clarithromycin. Dig Dis Sci. 1999;44:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Kusuhara H, Hirayama F, Matsuyuki H, Hisadome M, Ikeda Y. Evaluation of combined antibiotic-omeprazole therapies in Helicobacter pylori-infected Mongolian gerbils. J Gastroenterol. 1998;33:14-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Suzuki H, Mori M, Seto K, Nagahashi S, Kawaguchi C, Kai A, Akiba Y, Suzuki M, Suematsu M, Miura S. Ethanol intake preceding Helicobacter pylori inoculation promotes gastric mucosal inflammation in Mongolian gerbils. J Gastroenterol Hepatol. 1999;14:1062-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Takahashi S, Fujita T, Yamamoto A. Nonsteroidal anti-inflammatory drug-induced acute gastric injury in Helicobacter pylori gastritis in Mongolian gerbils. Eur J Pharmacol. 2000;406:461-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Tanigawa T, Kawamori T, Iimuro M, Ohta T, Higuchi K, Arakawa T, Sugimura T, Wakabayashi K. Marked enhancement by fish meal of Helicobacter pylori-induced gastritis in Mongolian gerbils. Jpn J Cancer Res. 2000;91:769-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |