Published online Feb 21, 2005. doi: 10.3748/wjg.v11.i7.1052
Revised: June 18, 2004
Accepted: July 17, 2004
Published online: February 21, 2005
AIM: To identify the gastrointestinal stromal tumors (GISTs) that are negative for CD117 expression by immunohistochemistry and to characterize their malignant potential.
METHODS: A total of 108 primary mesenchymal tumors of the gastrointestinal tract were screened to select CD117-negative tumors, from which KIT (exons 9, 11, 13, and 17) and PDGFRA (exons 10, 12, 14, and 18) were sequenced to identify GISTs. Tumor recurrence and distant metastasis were used as the criteria of malignancy.
RESULTS: The result showed that approximately 25% (29/108) of the gastrointestinal mesenchymal tumors were negative for CD117 and approximately 6% (7/108) of the tumors were CD117-negative GISTs. All these CD117-negative tumors had a mutated KIT and a wild-type PDGFRA. All CD117-negative GISTs with mutations at codons 557/558 of KIT had mitotic counts >10/50 high power field, and 75% (3/4) of them showed multiple recurrence or distant metastasis.
CONCLUSION: CD117-negative KIT mutated GISTs account for approximately 6% of the gastrointestinal mesenchymal tumors. Tumor recurrence or distant metastasis correlates to both the KIT mutations at codons 557/558 and the mitotic counts, but not to the tumor size.
- Citation: Tzen CY, Mau BL. Analysis of CD117-negative gastrointestinal stromal tumors. World J Gastroenterol 2005; 11(7): 1052-1055
- URL: https://www.wjgnet.com/1007-9327/full/v11/i7/1052.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i7.1052
Before our current understanding of the gastrointestinal stromal tumor (GIST), the term stromal tumor was originally introduced to describe mesenchymal tumors of the gastrointestinal (GI) tract that do not have features of Schwann cells or smooth muscle cells[1]. Subsequently, this term refers collectively to all mesencymal tumors regardless of the differentiation phenotype[2]. In 1998, Kindblom et al[3] reported that GIST expresses KIT tyrosine-kinase receptor, supporting its origin from a stem cell that differentiates into interstitial cells of Cajal. Since then, GISTs have been defined as KIT-expression mesenchymal tumors of the GI tract[4] regardless of whether there are co-expressed differentiation markers of myogenic phenotype (e.g., α-smooth muscle actin [SMA] or desmin) or neurogenic phenotype (e.g., S-100 and neuron-specific enolase). In this regard, the immunoreaction to a marker for KIT (i.e., CD117 positivity) becomes a requirement for the diagnosis of GISTs with very rare exceptions.
Gain-of-function mutation of KIT is believed to be the initial oncogenic step leading to the development of GIST[5]. Several investigators have shown that 52% to 92% of GISTs harbor KIT mutation[6-8] and KIT mutation is associated with aggressive behavior in GISTs[9,10]. A recent study also showed that approximately 35% of GISTs, lacking mutated MIT, have PDGFRA mutation[11].
Although the CD117 positivity by immunohistochemistry provides a simple and clear marker for the diagnosis, some authors believe that a few GISTs may not express CD117[12,13]. These tumors, if existed, are likely to be overlooked if the CD117 expression is a requirement for the diagnosis unless mutated KIT or PDGFRA genes are identified. In the present study, we screened primary mesenchymal tumors of the GI tract that were morphologically indistinguishable from GIST and sequenced the KIT and PDGFRA exons in all tumors that were negative for CD117. Then, we characterized the CD117-negative GISTs in terms of immunophenotype, mutation pattern, and clinical behavior.
A total of 108 primary mesenchymal tumors of the GI tract in the pathology archives of Mackay Memorial Hospital were coded as smooth muscle tumors (leiomyoma or leiomyosarcoma), schwannomas, fibromatoses, solitary fibrous tumors, inflammatory myofibroblastic tumors, gastrointestinal autonomic nerve tumors, stromal tumors or GISTs, during the period from 1995 to 2002.
For immunohistochemical staining, 5 μm representative sections of the specimens were deparaffinized with xylene and rehydrated in graded alcohols. Antibody against CD117 (1:50 dilution; Dako, Carpinteria, CA), S-100 protein (1:1500; Dako), desmin (1:50; Dako), and SMA (1:100, Dako) were commercially available. Immunoreaction was detected according to the manufacturer’s instructions (Ventana Medical Systems, Tucson, AZ). Staining for CD117 was considered negative if less than 5% of the tumor cells were weakly stained.
To isolate DNA from formalin-fixed tumors, representative paraffin blocks were cut at 8 μm using a clean disposable microtome blade. To ensure representative sampling, excess tissue was trimmed before sectioning. The first and the last sections from each ribbon were subjected to light microscopic examination after routine hematoxylin & eosin staining.
The paraffin sections were transferred directly into the PCR tubes and incubated in 300 μL of xylene at 25 °C for 5 min, pelleted at 12000 g for 5 min, re-suspended in 300 μL of absolute alcohol at room temperature, spun down, and lyophilized. The pellets were then processed using the Puregene DNA isolation kit (Gentra, Minneapolis, MN) according to the manufacturer’s instructions, which include proteinase K (300 μg/mL) digestion overnight at 55 °C. The final extracts were dissolved in TE buffer and kept at 4 °C for later use.
Four pairs of oligonucleotide primers were used to amplify exons 9, 11, 13, and 17 of KIT gene and exons of 10, 12, 14, and 18 of PDGFRA gene. The primer pairs to amplify KIT were 9R (5’-TGACATGGTCAATGTTGGAA-3’) and 9L (5’-AGCCAGGGCTTTTGTTTTCT-3’) for exon 9, 11R (5’-TGGAAAGCCCCTGTTTCATA-3’) and 11L (5’-CGTAATCGTAGCTGGCATGA-3’) for exon 11, 13R (5’-GCAAGAGAGAACAACAGTCTGG-3’) and 13L (5’-CATGCGCTTGACATCAGTTT-3’) for exon 13, and 17R (5’-TGAACATCATTCAAGGGTACTTTTG-3’) and 17L (5’-TTGAAACTAAAAATCCTTTGCAGGAC-3’) for exon 17. The primer pairs to amplify PDGFRA were 10R (5’-AGATGGTTTGAGAGATGGTACTGC-3’) and 10L (5’-GGACACAGTAGAGTCCAACAACGT-3’) for exon 10, 12F (5’-TCCAGTCACTGTCGCTGCTTC-3’) and 12R (5’-GCAAGGGAAAAGGGAGTCTT-3’) for exon 12, 14R (5’-CTCACTCTCATTCAAACCTATCAGC-3’) and 14L (5’-TCATACCCATCTCCTAACGGC-3’) for exon 14, and 18F (5’-ACCATGGATCAGCCAGTCTT-3’) and 18R (5’-TGAAGGAGGATGAGCCTGACC-3’) for exon 18.
PCR was carried out according to previously described procedures[11,14]. The PCR products were sequenced using the ABI PRISM BigDye terminator cycle sequencing ready reaction kit and ABI Prism 377 genetic analyzer (PE Applied Biosystems, Foster City, CA). All PCR products and independent duplicates were sequenced on both strands.
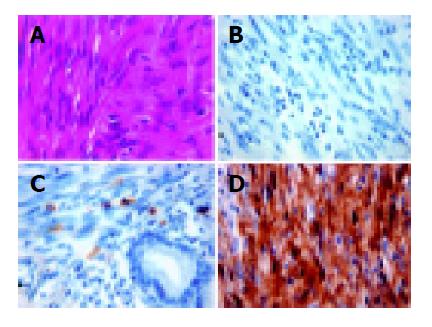
Of the 108 primary mesenchymal tumors of the GI tract examined, 79 tumors were positive for CD117 expression by immunohistochemistry, whereas 29 (Figures 1A and 1B) were negative for CD117, i.e., less than 5% of the tumor cells were weakly stained. All these CD117 negative tumors had positive mast cells in the adjacent areas as an internal control (Figure 1C). Among these CD117-negative tumors, 14 were myogenic (positive for SMA or desmin, negative for S-100), 4 were neurogenic (positive to S-100, negative for SMA and desmin), 9 were null-phenotypic (negative to both S-100 and SMA/desmin), and 2 showed dual differentiation (positive to both S-100 and SMA/desmin).
All these CD117-negative tumors were subjected to PDGFRA genomic DNA sequencing for the exons 10, 12, 14, and 18 as well as KIT genomic DNA sequencing for the exons 9, 11, 13, and 17. The result showed no detectable mutations in PDGFRA gene. However, 7 of them had KIT mutations, all of which were located in the exon 11 and consisted of 4 missense mutations and 3 in-frame deletion mutations. Codon 557 (tryptophan) and codon 558 (lysine) were most commonly affected, accounting for 57% (4/7) of the KIT mutations.
KIT mutations were identified in 5 of 9 null-phenotypic tumors, 1 of 4 neurogenic tumors (Figure 1D), 1 of 2 dual differentiated tumors and none (0/14) of the myogenic tumors. Fisher’s exact test showed a significant heterogeneity in KIT mutation frequency according to the tumor type (P = 0.0051). A closer look further showed that null-phenotypic tumors and myogenic tumors were more significantly different (P = 0.0037).
The clinical and histologic features of these CD117-negative GISTs are summarized in Table 1. Among these patients with CD117-negative GISTs, there were five men and two women, at an average age of 52.7 years (range, 44 to 69 years). Three tumors occurred in the stomach, two in small intestine, and two in rectum. Nearly all CD117-negative GISTs were composed of spindle tumor cells. The mitotic counts ranged from 2 to 367 per 50 HPF. Four of these tumors (#106, #133, #142, and #146) had mitotic count greater than 10/50 HPF. The tumor sizes ranged from 4.5 cm to 40 cm (range, 4.5 to 40 cm). Four tumors (#112, #142, #146, and #151) measured larger than 10 cm in greatest dimension, two (#106 and #133) measured 5-10 cm, and one (#23) smaller than 5cm. If tumor recurrence or metastasis was used as the criterion for malignancy, three cases (#133, #142, and #146) belonged to this category.
| Case Number | Age (yr) Sex Location | Tumor size1 Cell type MI2 | S-100 | SMA3 or desmin | Clinical behavior (follow-up months) |
| #23 | 52 Female Stomach | 4.5 cm Spindle MI = 2 | + | - | No evidence of disease after excision (24 mo) |
| #106 | 69 Female Stomach | 5.5 cm Spindle MI = 30 | - | - | No evidence of disease after excision (15 mo) |
| #112 | 44 Male Rectum | 13 cm Spindle MI = 7 | - | - | No evidence of disease after excision (12 mo) |
| #133 | 56 Male Rectum | 6 cm Spindle MI = 13 | - | - | Alive with local recurrence for 5 times (35 mo) |
| #142 | 45 Female Small intestine | 15 cm Spindle MI = 367 | - | - | Died of disease (48 mo); failed to Grivec treatment |
| #146 | 53 Female Small intestine | 17 cm Mixed MI = 100 | - | - | Alive with multiple metastasis (36 mo); remission after Glivec treatment |
| #151 | 50 Female Stomach | 40 cm Spindle MI = 3 | + | + | No evidence of disease after excision (17 mo) |
Acknowledging the fact that further studies are needed to fully understand the molecular basis of GIST, it would seem that gain-of-function mutation of KIT is one of the critical oncogenic steps in the tumor development. CD117 positivity may not correlate well to KIT mutation[12,13] because mutated KIT in some tumors has a low expression at protein level or the epitope of receptor has defects. Alternatively, fixation of the specimen and sources of the antibody (C-19 of Santa Cruz vs A4502 of DAKO) may influence the reaction, resulting in false negative staining by immunohistochemistry. In this study, all CD117-negative tumors had immunoreactive mast cells in their adjacent tissues, thus excluding the possibility of technical failure.
In this study, we screened a total of 108 mesenchymal tumors of the GI tract. The result showed that 29 (25%) of these tumors were negative for CD117 expression. Among them, seven harbored mutated KIT and none had mutated PDGFRA. About 57% KIT mutations in CD117-negative GISTs were missense mutations, causing a higher ratio of missense to deletion/insertion mutation than that of the CD117-positive GISTs, which was 23:77 in our series (unpublished observation). We could not explain the reason for this phenomenon.
Based on the immunoreactivity to S-100 and SMA/desmin, these 29 CD117-negative tumors could be divided into four groups. It is the current concept that GISTs are derived from stem cells that differentiate into interstitial cells of Cajal, which are positive for CD117 by immunohistochemistry. Therefore, GISTs without CD117 expression are probably better regarded as “null phenotypic” when immunostaining for SMA/S-100 is also negative, as opposed to the “prototypic” GISTs that are immunopositive for CD117. In this study, we also found that one of four CD117-negative and S-100-positive tumors harbored mutated KIT (Figure 1). Because patients with KIT-mutant tumors (regardless of their immunoreactivity to S-100) can benefit from imatinib treatment just like other GIST patients, it is better to classify this kind of tumor as GIST instead of KIT-mutant “nerve sheath tumor” at least for the therapeutic purpose. The myogenic group is distinct because none of the tumors in this group harbored KIT mutation, suggesting that true myogenic differentiation and KIT mutation are mutually exclusive. However, when the true myogenic tumors were excluded, the heterogeneity in KIT mutation became insignificant (P = 0.765, Fisher’s exact test). This finding supports the argument[10] for differentiation of true smooth muscle tumors from GIST, and argues against differentiating other tumors from GIST.
So far, all the grading systems for GIST are derived from the observation on the CD117-positive tumors. Most of these systems, such as the NIH Consensus Guidelines for grading[4], give weight to both mitotic count and tumor size. According to this system, there were one low-risk tumor (#23) and six high-risk tumors in our CD117-negative GISTs. In contrast, some grading systems such as the three-tiered grading scheme[15] emphasize mitotic count and cytologic feature but not tumor size. Based on this scheme, there were two low-risk tumors (#23 and #151), one intermediate-risk tumor (#112), and four high-risk tumors (#106, #133, #142, and #146). Because three of the four high-risk tumors in this grading system (#133, #142, and #146) showed either multiple recurrence or distant metastasis, the three-tiered grading scheme correlates better with the clinical behavior of CD117-negative GISTs. This finding suggests that tumor size might not be an important prognostic factor in CD117-negative GISTs, as opposed to CD117-positive tumors. In fact, only two of four tumors with their sizes >10 cm in this study were malignant and the largest tumor (case #151) measuring 40 cm was microscopically of low risk and clinically benign.
Mutations at certain positions may be associated with aggressive tumor behavior. All CD117-negative GISTs with mutations at codons 557/558 of KIT were high-risk in the three-tiered grading scheme[15], and vice versa. Therefore, 75% (3/4) of them showed multiple recurrence or distant metastasis. A similar observation was also made in CD117-positive GISTs with mutations at codons 557/558 occurred in 79% (11/14) of malignant tumors[10]. In contrast, mutations at codons 557/558 only accounted for 34% (15/34) of all GISTs with exon 11 genomic mutations[6].
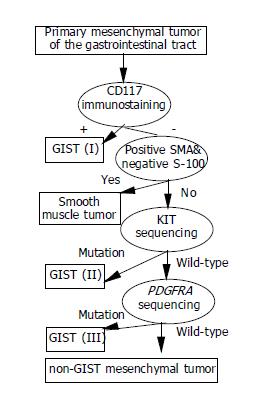
In conclusion, CD117-negative GISTs do exist (Figure 2), accounting for approximately 6% of the gastrointestinal mesenchymal tumors. They are immunohistochemically different from smooth muscle tumors, which are positive for SMA/desmin but negative for S-100 and CD117. A final diagnosis of CD117-negative GIST depends on the presence of mutated KIT or PDGFRA. Although generalizations about the malignant potential of CD117-negative GISTs cannot be made, our limited cases show a correlation to KIT mutations at codons 557/558 and mitotic counts, but not to tumor size.
The authors thank Mr. Zon-Darr Huang for technical assistance, Dr. Meifen Kung for statistical analysis, and Ms. Ching-Hui Chen for secretarial expertise.
| 1. | Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983;7:507-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 560] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 2. | Schaldenbrand JD, Appelman HD. Solitary solid stromal gastrointestinal tumors in von Recklinghausen's disease with minimal smooth muscle differentiation. Hum Pathol. 1984;15:229-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 59] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152:1259-1269. [PubMed] |
| 4. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2149] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 5. | Heinrich MC, Rubin BP, Longley BJ, Fletcher JA. Biology and genetic aspects of gastrointestinal stromal tumors: KIT activation and cytogenetic alterations. Hum Pathol. 2002;33:484-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 302] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 6. | Rubin BP, Singer S, Tsao C, Duensing A, Lux ML, Ruiz R, Hibbard MK, Chen CJ, Xiao S, Tuveson DA. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res. 2001;61:8118-8121. [PubMed] |
| 7. | Lasota J, Wozniak A, Sarlomo-Rikala M, Rys J, Kordek R, Nassar A, Sobin LH, Miettinen M. Mutations in exons 9 and 13 of KIT gene are rare events in gastrointestinal stromal tumors. A study of 200 cases. Am J Pathol. 2000;157:1091-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 239] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | Hirota S, Nishida T, Isozaki K, Taniguchi M, Nakamura J, Okazaki T, Kitamura Y. Gain-of-function mutation at the extracellular domain of KIT in gastrointestinal stromal tumours. J Pathol. 2001;193:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Ernst SI, Hubbs AE, Przygodzki RM, Emory TS, Sobin LH, O'Leary TJ. KIT mutation portends poor prognosis in gastrointestinal stromal/smooth muscle tumors. Lab Invest. 1998;78:1633-1636. [PubMed] |
| 10. | Lasota J, Jasinski M, Sarlomo-Rikala M, Miettinen M. Mutations in exon 11 of c-Kit occur preferentially in malignant versus benign gastrointestinal stromal tumors and do not occur in leiomyomas or leiomyosarcomas. Am J Pathol. 1999;154:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 384] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 11. | Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1712] [Cited by in RCA: 1723] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 12. | Riddell RH, Petras RE, Williams GT, Sobin LH. Mesenchymal tumors In: Rosai J, Sobin LH eds. Tumors of the Intestine, Atlas of Tumor Pathology. Armed Forces Institute Pathol. 2002;325-394. |
| 13. | Kindblom LG (2002) Epidemiology, pathology, and diagnostic criteria of GIST. Available from: http: //www.glivec.com/pdf/X0061_London.pdf). |
| 14. | Miettinen M, Kopczynski J, Makhlouf HR, Sarlomo-Rikala M, Gyorffy H, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the duodenum: a clinicopathologic, immunohistochemical, and molecular genetic study of 167 cases. Am J Surg Pathol. 2003;27:625-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 280] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 15. | Singer S, Rubin BP, Lux ML, Chen CJ, Demetri GD, Fletcher CD, Fletcher JA. Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol. 2002;20:3898-3905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 310] [Article Influence: 13.5] [Reference Citation Analysis (0)] |