INTRODUCTION
In acidic stomach lumen and ongoing acid-peptic digestive process, adequate mucosal blood flow is one of the essential mechanisms to maintain mucosal integrity since this blood flow removes diffused acid and delivers energy to support the normal mucosal function[1]. Likewise, acute stress usually results in ischemia and the following ulcerations[2]. Besides, gastric mucosal blood flow is diminished in patients with active gastric ulcer compared to that of healed subjects[3]. Endothelins are the family of three homologous 21-aminoacid peptides in terms of endothelin-1 (ET-1), ET-2 and ET-3. ET was originally found in the endothelium of blood vessels with very potent vasoconstriction ability[4-6]. The submucosal injection of ET-1 in gastric wall results in mucosal injury since its integrity is destroyed by the diminished blood flow[7]. In addition, the higher plasma ET-1 level is found in gastric ulcer patients. Perhaps ET-1 is one of the mediators contributing to the pathogenesis of peptic ulcer[8]. Nitric oxide (NO) is a free radical molecule generated by three well known NO synthases (neuronal, endothelial and inducible-NOS) via electron transfer step[9]. NO behaves as an endogenous vasodilator to modulate stomach mucosal blood flow in maintaining its integrity[10]. NO pathway also exhibits other gastrointestinal functions including epithelial permeability, motility and even inflammation. iNOS is highly expressed in response to epithelial cell injury, apoptosis, host immune response and perpetuation of inflammatory responses[9-11]. It means that iNOS-derived NO generation occurs at a greater order in these events than that of other isoforms[9]. Accordingly, over expression of iNOS in gastrointestinal mucosa is one of the factors leading to mucosal injury[12]. Currently, Helicobacter pylori (H pylori) colonization has been considered as the most important etiology of peptic ulcer diseases[13]. H pylori infection produces many peptides and inflammatory cytokines in turn leading to major pathophysiological changes in the spectra ranging from gastritis to cancer[14,15]. Eradication treatment has been strongly recommended to all H pylori related duodenal ulcer (DU) patients[13,15]. Whether H pylori infection also has an impact on the releasing of ET-1 and NO in DU patients is unknown. If this impact exists, an effective H pylori eradication should attenuate these released products upon the disease course. The aim of this study was to determine whether the plasma ET-1 and NO levels were associated with H pylori infection in DU patients, especially the effect of H pylori eradication.
MATERIALS AND METHODS
This study was conducted between August 2001 and July 2002 as a single-center trial. Inclusion criteria were as follows: patients of both sexes, age between 20 and 80 years, presenting with dyspeptic symptoms, with no H pylori eradication history or obvious gastroesophageal reflux symptoms. According to the study protocol, only the H pylori-infected patients with an active ulcer crater in the duodenal bulb with a minimal size over 2 mm diagnosed based on endoscopy were eligibly enrolled. During the diagnostic endoscopy, ulcer size was measured while biopsy specimens were simultaneously obtained from the antrum and body for a rapid urease test and histological examination to determine H pylori colonization. When both methods were positive for H pylori, H pylori infection was established. All patients treated previously with ulcerogenic or acid-reducing drugs within 2 wk prior to endoscopy or failed in H pylori eradication were excluded. Other exclusion criteria included pregnancy, concomitant gastric or prepyloric ulcer, recent DU-related bleeding, inability to suspend any ulcerogenic drugs during the study, or surgery on the upper gastrointestinal tract. For comparison, age and sex-matched healthy controls were enrolled from healthy subjects who received a paid physical check up including routine endoscopy in this hospital. Neither dyspepsia complaint nor main endoscopic finding was acknowledged to be a control. Because many diseases are associated with abnormal plasma ET-1 and NO levels, neither the studied patients nor the control subjects had acute pancreatitis, and chronic pulmonary, liver, renal, cardiovascular, or cerebrovascular diseases[6,16]. This study was approved by the Institutional Review Board of Taipei Veterans General Hospital, and informed consent was obtained from all cases prior to the study.
HP eradication and follow-up
After each diagnostic endoscopy, plasma samples of all infected DU patients were collected in the fasting state and stored at -80 °C until measurement. The patients received H pylori-eradicated triple therapy consisting of rabeprazole 20 mg twice daily, amoxicillin 1000 mg twice daily and metronidazole 500 mg twice daily for one week and then acid-reducing therapy of rabeprazole 20 mg once daily for another one month. Afterwards, they were asked back on the appointed date for the second endoscopic reassessment of DU and H pylori states one month after the final regimen was completed. During this period, neither antibiotics nor acid-reducing agent was allowed for the studied subjects except for antacids in occurrence of dyspepsia. H pylori eradication was defined when both urease test and histologic examination were negative.
Plasma endothelin-1 and nitric oxide level measurement
Plasma ET-1 level was measured with a commercial enzyme immunoassay kit [Endothelin-1 (human), Cayman, Ann Arbor, MI, USA] while plasma NO activity was determined by measuring the nitrate/nitrite level (Nitrate/Nitrite, Cayman). The technician responsible for the NO and ET-1 measurement was not aware of the current status of all studied subjects.
Statistical analysis
Results were expressed as mean±SE. Numerical data were analyzed using either Student’s t test, Wilcoxon signed rank test or Kruskal-Wallis one-way analysis of variance (ANOVA). Linear regression was used to study the correlation coefficient between two variables. P value less than 0.05 was considered statistically significant.
RESULTS
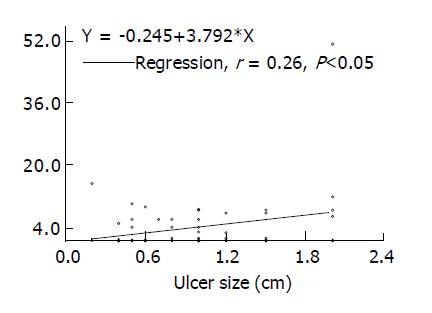
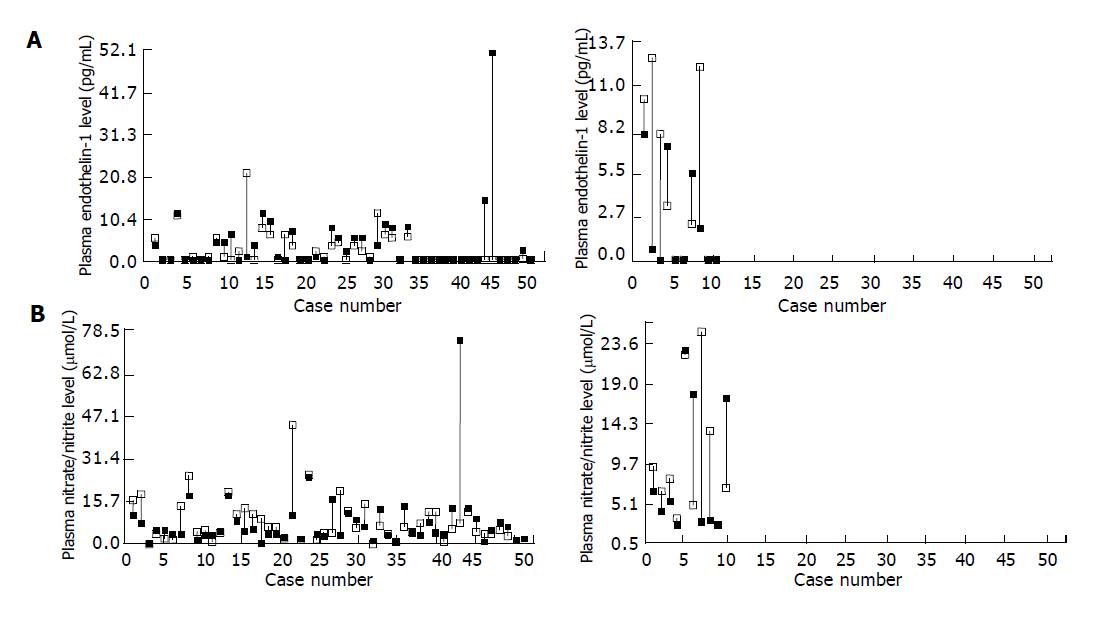
A total of 66 active DU patients (M/F: 46/20, age: 22-68 years) meeting our defined criteria were consecutively enrolled. Sixty of them (90.9%) finished the whole procedure per protocol, 52 (86.7%) patients had healed DU in the follow-up, whereas successful H pylori eradication was achieved in 50 (83.3%) subjects. Meanwhile 37 healthy subjects were enrolled for comparison during this period. Seventeen of them (M/F: 12/5, age: 32-58 years) were H pylori negative and 20 (M/F: 13/7, age: 38-54 years) were H pylori positive. The plasma ET-1 levels in 17 H pylori-negative controls, 20 H pylori-positive controls and 60 active DU patients were 0.89±0.54, 0.3±0.2 and 3.59±0.96 pg/mL, respectively (P<0.01). While the plasma nitrate/nitrite levels in three studied groups were 5.27±0.68, 6.39±0.92 and 8.55±0.71 µmol/L, respectively (P<0.05). The correlation coefficients of plasma ET-1 and nitrate/nitrite levels against ulcer sizes in 60 DU patients were 0.26 (P<0.05) and 0.15 (NS), respectively (Figure 1). Figure 2 depicts the change of plasma ET-1 levels in DU patients after triple therapy. Among the 50 H pylori-eradicated subjects, ET-1 level diminished in the follow-up (3.65±0.54 vs 2.64±0.55 pg/mL, P<0.01), whereas this decline was not observed in 10 subjects without H pylori eradication (2.39±1.20 vs 4.94±1.20 pg/mL, NS). Figure 2 shows the change of plasma nitrate/nitrite levels in these DU patients. Nitrate/nitrite level in the 50 H pylori- eradiacted subjects elevated in the follow-up (8.16±0.84 vs 11.41±1.42 µmol/L, P<0.05). However, this elevation was not found in 10 patients without H pylori eradication (8.81±2.540 vs 11.60±2.85 µmol/L, NS). The correlation coefficients of plasma ET-1 levels against nitrate/nitrite levels before and after triple treatment were -0.46 and -0.068 in these 10 DU patients, respectively (NS), and were -0.16 and 0.13 in 50 H pylori-eradicated DU patients.
Figure 1 Measured plasma endothelin-1 levels and ulcer sizes in 60 patients with active ulcer crater.
Figure 2 Change of plasma ET-1 levels (A) and nitrate/nitrite levels (B) in duodenal ulcer patients before (■) and after (□) H pylori eradication treatment.
DISCUSSION
In our study, the H pylori eradication rate in active DU patients undergoing this triple therapy was 83.3%. This result is comparable with others as well as that in our previous study using proton pump inhibitor based treatment[13,17-19]. Furthermore, the H pylori infection in all healed subjects was eradicated. H pylori infection induces inflammatory responses in mucosa including increased cytokines, activation of inflammation and stimulates the production of prostaglandins, gastrin and somatostatin, etc.[14]. The imbalance of these factors determines the ulcer occurrence or recurrence. Consequently, various cytokine and peptide responses have a significant change after effective H pylori eradication, e.g., the decline of various cytokines and gastrin production[20-22].
ET-1 appears to be a candidate in the mechanisms leading to gastric mucosal injury. For example, increased intraluminal acid and the following ulcerations are induced after exogenous ET-1 treatment[7]. Furthermore, endogenous ET-1 is a powerful mediator of stress-evoked gastric mucosal damage in rats[23,24]. Masuda et al[8] indicated that the plasma ET-1 level in patients with active gastric ulcer is higher than that in these healed subjects and controls. Besides, the ulcer areas of these subjects have a significant correlation with plasma ET-1 levels (r = 0.7). Similarly, our study pointed out that ET-1 level in active DU patients is also higher than that in controls, and effective eradication can restore its level. The ulcer sizes exhibit a weak correlation with plasma ET-1 levels. Taking these observations into consideration, we suggest that the increased ET-1 production is one of the mechanisms leading to peptic ulcer formation, regardless of ulcer location.
ET-positive cells are found in vascular smooth muscle, gastric epithelium and stomach smooth muscle[25]. It remains controversial about the role of H pylori in the production of ET-1 in peptic ulcer patients. Mori et al[26] observed that gastric endogenous ET-1 plays a role in ammonia-induced gastric mucosal injury mediated via muscarine and ETA receptors. Since ammonia is the main product of H pylori, it seems that ET-1 is closely related to H pylori elicited mucosal injury. Our control study found that H pylori infection could not produce ET-1. In contrast, once DU occurs, more ET-1 is produced. H pylori eradication can reduce ET-1 production. These data suggest that ET-1 is not the only decisive factor for H pylori-related DU, other pathways are involved in the pathogenesis of DU.
Sufficient mucosal blood flow is dependent upon the balance between the endothelial released substances increasing blood flow, vasodilatation and anti-aggregatory activity and the substances reducing blood flow and promoting platelet aggregation[8]. Evidence indicates that NO acts as one of the endogenous vasodilators to regulate gastroduodenal mucosal blood flow and to maintain its integrity and defense[9,27]. A good correlation exists between blood/gastric mucosal nitrate/nitrite concentration and gastric iNOS activity[28]. The increased NO production via iNOS in gastric mucosa of stressful rats is believed to lead to mucosal lesions[28]. Histologically, iNOS-positive cells are not confined to the vascular smooth muscle only[29]. The higher nitrate/nitrite level in DU patients is likely in this example. Similarly, mucosal NO activity is also higher in patients with gastric ulcer[25]. We suggest that more nitrate/nitrite production in DU patients is the feedback protective response to stress or ulceration. However, we believe that this is not a direct response to increased ET since no correlation exists between the two parameters.
Our study showed that H pylori infection in control subjects could not produce more nitrate/nitrite unless presented with active DU, we suggest that significant NO production is not due to H pylori infection since its eradication could not diminish nitrate/nitrite level.
Since some of the degraded H pylori extracts in duodenum are the free amino acid residues presenting as a potent inhibitor of NO system, their blockage of gastroduodenal NO seems to be one of the mechanisms underlying the decreased mucosal alkaline secretory capacity in DU patients[30]. An animal study also confirmed the interference in mucosal NO synthase if H pylori extract is treated[31]. Accordingly, the inhibition of NO system by H pylori infection or its product leads to ulcerations. It is unknown whether DU patients showing ulcer healing and H pylori eradication can restore duodenal mucosal alkaline secretion. Future studies are likely needed to confirm this putative mechanism. In conclusion, both plasma ET-1 and nitrate/nitrite levels increase in active DU patients. After effective H pylori eradication, DU healing is associated with diminished blood ET-1 level and elevated nitrate/nitrite activity. Ulcer processing rather than H pylori infection alone is likely to determine the changed activity of these vasomotor substances.