Published online Feb 21, 2005. doi: 10.3748/wjg.v11.i7.1018
Revised: August 27, 2004
Accepted: October 8, 2004
Published online: February 21, 2005
AIM: To estimate the detectability of anomalous connection in pancreatobiliary disease (ACPBD) cases, measuring gallbladder wall blood flow (GWBF).
METHODS: In the retrospective study, we enrolled 42 subjects with gallbladder wall thickening. GWBF velocity was determined as an average value of the peak velocity of color signals on the gallbladder wall, three times in each case. Based on the findings on endoscopic ultrasonography (EUS) or endoscopic retrograde cholangiopancreatography (ERCP), the 42 subjects were divided into 11 cases with ACPBD and 31 cases without ACPBD. In the prospective study, the subjects were 92 cases with gallbladder wall thickening. Using the cut-off level of the flow velocity obtained in the retrospective study, the usefulness of measuring GWBF velocity in diagnosing ACPBD was evaluated.
RESULTS: In the retrospective study, imaging of GWBF was obtained in 40 of the 42 subjects. The mean GWBF velocity of the ACPBD cases was 29.4±3.9 cm/s (mean±SD), which was significantly different (P<0.0001; 95% CI 5.48-13.2) from that of the without ACPBD cases (20.1±5.9 cm/s). Based on this result, we prepared a receiver operating characteristic curve, and the cut-off level appropriate for diagnosing ACPBD was estimated to be 25 cm/s. In the prospective study, GWBF was detected in 86 of the 92 subjects. Based on the EUS or ERCP findings, the 92 subjects were divided into 15 cases with ACPBD and 77 cases without ACPBD. When a cut-off level of 25 cm/s was employed, ACPBD could be diagnosed with a sensitivity of 87.0% (13/15) and a specificity of 87.3% (62/71).
CONCLUSION: Meas urement of GWBF velocity, which is less invasive and provides objective values, is very useful for diagnosing ACPBD prior to the development of malignant tumors in cases with gallbladder wall thickening.
- Citation: Kawashima H, Hirooka Y, Itoh A, Hashimoto S, Itoh T, Hara K, Kanamori A, Ohmiya N, Niwa Y, Goto H. Use of color Doppler ultrasonography in the diagnosis of anomalous connection in pancreatobiliary disease. World J Gastroenterol 2005; 11(7): 1018-1022
- URL: https://www.wjgnet.com/1007-9327/full/v11/i7/1018.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i7.1018
The junction of the common bile duct and the pancreatic duct is crucial for sphincteric control of bile and pancreatic juice drainage. Anomalous connection in pancreatobiliary disease (ACPBD) is a condition in which the junction between the common bile duct and the pancreatic duct is located outside the duodenal wall[1]. Therefore, the action of the sphincter of Oddi does not affect the junction; the communication between these ducts is always present, and so pancreatic juice flows into the biliary tract through the communication. ACPBD has attracted attention because of its frequent association with biliary malignancy[2-4]. Thus it is important for ACPBD cases to be detected more efficiently before biliary malignancy develops. It has been reported that gallbladder wall thickening is characteristic of ACPBD on abdominal ultrasonography. But these findings are rather subjective. To efficiently and objectively detect a low incidence of ACPBD, high-risk patients should be detected from cases with these findings. Endoscopic ultrasonography (EUS) is carried out in selected cases to detect ACPBD in the subjects with gallbladder wall thickening, and then endoscopic retrograde cholangiopancreatography (ERCP) is performed to confirm the existence of ACPBD. However, no examination procedure has been established[5] to detect non-invasively and efficiently precancerous ACPBD.
On the other hand, we reported the usefulness of gallbladder wall blood flow (GWBF) velocity in the diagnosis of gallbladder disorders; a higher velocity of gallbladder wall blood flow suggested malignant potential[6-9]. In this study, we investigated the usefulness of measuring GWBF velocity in the diagnosis of ACPBD in patients with gallbladder wall thickening to facilitate the more efficient detecting of ACPBD.
For the present study, 3 mm or greater gallbladder wall thickening was considered indicative of potentially underlying ACPBD[5]. Forty-two consecutive subjects with gallbladder wall thickening who visited Nagoya University Hospital between October 1996 and September 1998 were examined in this study. They included 28 males and 14 females with a mean age of 50.5±2.47 (mean±SE) years. The subjects did not have any symptoms, and ultrasonography on a health checkup showed gallbladder wall thickening. Patients with acute cholecystitis, hepatitis, chronic liver disease, or hepatic neoplasm were excluded, because it has been reported that patients with these disorders show a specific type of gallbladder wall blood flow[7,10,11], with a high flow velocity. In each subject, fast Fourier transform analysis of the flow velocity was performed using imaging of GWBF obtained by color Doppler-guided spectral analysis.
After GWBF velocity was measured, EUS was performed to investigate the presence or absence of ACPBD in all patients. In diagnosing ACPBD by EUS, we followed the course of the distal common bile duct and the pancreatic duct within the pancreatic parenchyma up to the level of the papilla, as described in previous studies[12]. Ten cases with pancreatobiliary connection in the pancreatic parenchyma were regarded as having ACPBD, whereas 28 cases in whom the common bile duct ran through the duodenal wall without connecting with the pancreatic duct were regarded as being free from ACPBD. In 4 cases in whom the distal common bile duct could not be evaluated by EUS, we considered diagnosis impossible. The echoendoscopes used in the present study were the EG-3630UR (Pentax, Tokyo, Japan), GF-UM240 and GF-UM2000 (Olympus, Tokyo, Japan).
In the cases in whom EUS suggested anomalous connection and in those in whom diagnosis was impossible, ERCP was performed to evaluate the presence or absence of ACPBD for definitive diagnosis. In the 10 cases in whom EUS suggested ACPBD, ERCP confirmed this finding. In 1 of the 4 cases in whom diagnosis was impossible on EUS, ACPBD was detected, but not in the other 3 cases. The diagnostic criterion for ACPBD by ERCP is a common channel longer than 15 mm. Moreover, for more definite proof of ACPBD, it is best to confirm that the contractile segment of the common channel ends well below the common channel[4]. Be summarized as follows, the 42 subjects were divided into 11 cases with ACPBD and 31 cases without ACPBD. In 5 of the 11 cases with ACPBD, dilatation of the bile duct was observed, but not in the other 6 cases. In the 11 cases with ACPBD, surgery was performed, and pathological findings confirmed gallbladder cancer in 2 cases, hyperplasia of the gallbladder in 5 cases, and gallbladder adenomyomatosis in 4 cases.
GWBF velocity was measured as previously described. The threshold and the mode of color Doppler sensitivity were adjusted to obtain the most sensitive color signal without aliasing. When the image of the cystic artery was obtained, we evaluated the flow of the cystic artery in the gallbladder body close to the hepatic bed. The values of peak flow velocity were measured at three points of the artery in the gallbladder, close to the hepatic bed, and were averaged. For velocity measurement, angle correction was made at that position. We tried to obtain a measurement of velocity less than 60 degrees in Doppler angle[7]. The HDI 3000 and 5000 (ATL, Philips, USA) and a 5 MHz convex or linear probe were used to measure GWBF velocity.
We compared GWBF velocity between the cases with ACPBD and those without ACPBD, and estimated the cut-off value of GWBF velocity for diagnosing ACPBD in cases with gallbladder wall thickening. In the cases with ACPBD, we examined GWBF velocity with respect to gallbladder disorders and the presence or absence of dilatation of the bile duct.
In the prospective study, the subjects were consecutive 92 cases with gallbladder wall thickening who visited our hospital between October 1998 and September 2003, and to simplify this study we excluded cases such as acute cholecystitis, hepatitis, chronic liver disease, or hepatic neoplasm as mentioned above. Using the cut-off level of the flow velocity obtained in the retrospective study, the usefulness of measuring GWBF velocity in diagnosing ACPBD was evaluated.
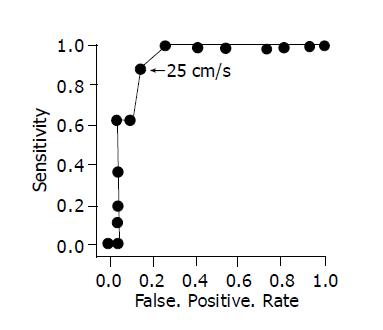
Analysis of unpaired t-test and ANOVA (analysis of variance) were used for statistical analysis of the data. Probability (P) values of less than 0.05 were considered significant. All values are expressed as the mean±SD. To determine the cut-off level of flow velocity, we prepared a receiver operating characteristic curve (ROC curve) based on the results of the retrospective study.
In the retrospective study, imaging of GWBF was obtained in 40 (95%) of the 42 subjects. In the ACPBD group, the peak mean GWBF velocity was 29.4±3.9 cm/s, and images of wall blood flow were obtained in all 11 cases. The mean flow velocity of each gallbladder disorder was as follows: in carcinoma (2 cases) it was 34.1±2.5 cm/s, in hyperplasia (5 cases) 30.1±3.2 cm/s, and in adenomyomatosis (4 cases) 26.7±2.0 cm/s. There was a significant difference in GWBF velocity between the patients with gallbladder cancer and those with adenomyomatosis (P = 0.0392 by ANOVA). However, there were no significant differences among the other disorder groups. There was no significant difference in GWBF velocity between the 5 ACPBD patients with dilatation of the bile duct and the 6 ACPBD patients without dilatation of the bile duct.
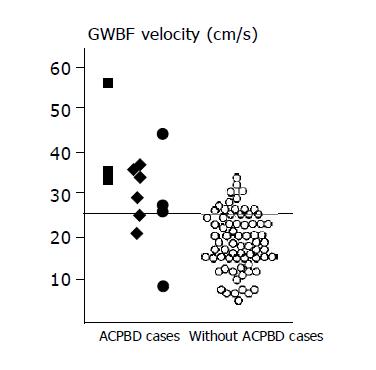
In the without ACPBD group, the mean GWBF velocity was 20.1±5.9 cm/s and images of GWBF were obtained in 29 (94%) of the 31 cases. The blood flow velocity within the gallbladder wall in the ACPBD group was significantly higher than that in the without ACPBD group (P<0.0001; 95% CI 5.48 - 13.2 = (Table 1). When preparing an ROC curve based on these results, the cut-off level of GWBF velocity appropriate for diagnosing ACPBD was estimated to be 25 cm/s (Figure 1). When the cut-off level of the GWBF velocity was set at 25 cm/s, the sensitivity and specificity for ACPBD by GWBF velocity were 90.9% (10/11) and 89.7% (26/29) respectively.
When GWBF close to the peritoneal cavity was compared with that close to the hepatic bed in subjects in whom images of wall blood flow were obtained, there was no significant difference in the flow between the two regions in any case.
In 86 (93%) of the 92 subjects, GWBF could be detected. EUS suggested ACPBD in 13 cases; there was no anomalous connection in 74 cases, and diagnosis was impossible in 5 cases. In 2 of the 5 cases, ERCP verified ACPBD, whereas there was no anomalous connection in the other 3 cases. Briefly, the subjects consisted of 15 cases with ACPBD and 77 cases without ACPBD. GWBF velocity could be measured in all cases with ACPBD and in 71 (92.2%) of the 77 cases without ACPBD. Twenty-eight cases showed a GWBF velocity of more than 25 cm/s. The sensitivity and specificity of GWBF velocity in the diagnosis of ACPBD were 86.7% (13/15) and 87.3% (62/71) respectively (Figure 2). In 7 of the 15 cases with ACPBD, dilatation of the bile duct was observed, but not in the other 8 cases.
In all cases with ACPBD, surgery was performed. Pathological findings confirmed gallbladder cancer in 3 cases, hyperplasia of the gallbladder in 8 cases (Figure 3), and gallbladder adenomyomatosis in 4 cases. Remaining 77 cases are under serial examination every 6 mo (6 from 66, mean 32.4±14.8 mo), and there have been no morphological change of the gallbladder.
In cases with ACPBD, malignant tumors of the biliary system develop at a young age, showing a poor prognosis in many patients[2-4]. Therefore, it is important to detect ACPBD before the development of malignant tumors. Gallbladder wall thickening and dilatation of the common bile duct on ultrasonography are characteristic of ACPBD, and a study has reported that ACPBD was observed in 2.9% of patients with gallbladder wall thickening and in 23% of patients with dilatation of the common bile duct[5]. To evaluate gallbladder wall thickening is rather subjective, so objective diagnostic criteria may be needed. To efficiently and objectively detect a low incidence of ACPBD, high-risk patients should be detected from cases with these findings. In cases with dilatation of the bile duct on abdominal ultrasonography, not only ACPBD but also malignant tumors of the bile duct or pancreas must be ruled out, and secondary examination should be aggressively performed. Therefore, we considered it important to efficiently detect cases with ACPBD from cases with gallbladder wall thickening, and this study investigated cases with gallbladder wall thickening regardless of the presence or absence of dilatation of the common bile duct. A study has indicated that GWBF velocity is high in patients with symptomatic acute cholecystitis[7,11]; therefore, these patients were excluded from this study. When gallbladder wall thickening persisted after improvement in symptoms, GWBF velocity was measured, and these patients were enrolled in this study. Hayakawa et al[7] have reported that gallbladder cancer could be detected with a sensitivity and specificity of 100% and 96% respectively when the cut-off value of GWBF velocity was set at 30 cm/s. When this cut-off value (30 cm/s) was applied to the results of this retrospective study, GWBF velocity was 30 cm/s or more in the 2 ACPBD cases with gallbladder cancer; however, GWBF velocity was less than 30 cm/s in 7 (77.8%) of the 9 patients with ACPBD prior to the development of cancer. Therefore, we prepared an ROC curve based on the results of the retrospective study to establish a cut-off value appropriate for the diagnosis of ACPBD, and established the cut-off value at 25 cm/s.
In our prospective study, we employed this cut-off value (25 cm/s), and investigated the usefulness of measuring GWBF velocity in the diagnosis of ACPBD. Measurement of GWBF velocity facilitated diagnosis in 13 (86.7%) of the 15 cases with ACPBD and in 7 (87.5%) of the 8 cases without dilatation of the bile duct. In this study, no patients without ACPBD had gallbladder cancer.
Previously, cholangiography such as ERCP has been used for the definitive diagnosis of ACPBD; however, EUS also facilitates accurate diagnosis with less invasiveness, and may be more useful for secondary examination[12]. In this study, the presence or absence of ACPBD could be evaluated by EUS in 136 (93.8%) of the 145 cases. However, diagnosis was impossible in 6.2% of the cases (3 (11.5%) of the 26 cases with ACPBD, 6 (5.0%) of the 119 cases without ACPBD); in these cases, the distal common bile duct could not be evaluated up to the level of the papilla.
In the 26 cases with ACPBD, GWBF velocity was investigated with respect to gallbladder disorders. The mean GWBF velocity was 39.0±10.4 cm/s in 5 cases with carcinoma, 29.8±4.6 cm/s in 13 cases with hyperplasia, and 26.5±9.6 cm/s in 8 cases with adenomyomatosis. There was a significant difference in GWBF velocity between the patients with gallbladder cancer and those with adenomyomatosis (P = 0.0296 by ANOVA). It has been reported that adenomyomatosis of the gallbladder frequently develops in cases with ACPBD[13,14]. In this study, 8 (30.8%) of the 26 cases with ACPBD had adenomyomatosis. When the mean GWBF velocity was compared between the 8 cases with ACPBD and adenomyomatosis of the gallbladder and the 11 cases with adenomyomatosis of the gallbladder without ACPBD in whom surgery and pathological diagnosis were performed for various reasons, the mean value (26.5±9.6 cm/s) in the former cases was significantly higher than that (18.4±5.3 cm/s) in the latter cases (P = 0.029; 95% CI 0.947-15.4). In 21 cases, excluding the cases with gallbladder cancer from the 26 patients with ACPBD, the mean GWBF velocity was 28.6±6.9 cm/s, which is significantly higher than that (19.3±6.4 cm/s) in the 100 cases without ACPBD (P<0.0001; 95% CI 6.18-12.3). Some studies have reported that the Ki-67 labeling index is high in the gallbladder wall of cases with ACPBD[15], and that immunostaining shows a high level of TNFα[16]. These findings suggest that cell proliferation is enhanced in the gallbladder wall of cases with ACPBD. This may contribute to a higher GWBF velocity in cases with ACPBD before the development of malignant tumors.
In this study, the mean GWBF velocity was 32.0±9.2 cm/s in the 12 ACPBD cases with dilatation of the bile duct and 29.3±8.1 cm/s in the 14 ACPBD cases without dilatation of the bile duct; there was no significant difference (P = 0.43; 95% CI -4.26-4.70).
Measurement of GWBF velocity is very useful for efficiently detecting patients with ACPBD from those with gallbladder wall thickening before the development of malignant tumors, since this non-invasive simple procedure provides objective values. In conclusion, GWBF measurement is useful in detecting potentially malignant cases out of cases with gallbladder wall thickening.
| 1. | Suda K, Miyano T, Konuma I, Matsumoto M. An abnormal pancreatico-choledocho-ductal junction in cases of biliary tract carcinoma. Cancer. 1983;52:2086-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Kinoshita H, Nagata E, Hirohashi K, Sakai K, Kobayashi Y. Carcinoma of the gallbladder with an anomalous connection between the choledochus and the pancreatic duct. Report of 10 cases and review of the literature in Japan. Cancer. 1984;54:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Yamauchi S, Koga A, Matsumoto S, Tanaka M, Nakayama F. Anomalous junction of pancreaticobiliary duct without congenital choledochal cyst: a possible risk factor for gallbladder cancer. Am J Gastroenterol. 1987;82:20-24. [PubMed] |
| 4. | Kimura K, Ohto M, Saisho H, Unozawa T, Tsuchiya Y, Morita M, Ebara M, Matsutani S, Okuda K. Association of gallbladder carcinoma and anomalous pancreaticobiliary ductal union. Gastroenterology. 1985;89:1258-1265. [PubMed] |
| 5. | Yamao K, Mizutani S, Nakazawa S, Inui K, Kanemaki N, Miyoshi H, Segawa K, Zenda H, Kato T. Prospective study of the detection of anomalous connections of pancreatobiliary ducts during routine medical examinations. Hepatogastroenterology. 1996;43:1238-1245. [PubMed] |
| 6. | Hirooka Y, Naitoh Y, Goto H, Furukawa T, Ito A, Hayakawa T. Differential diagnosis of gall-bladder masses using colour Doppler ultrasonography. J Gastroenterol Hepatol. 1996;11:840-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Hayakawa S, Goto H, Hirooka Y, Itoh A, Taki T, Watanabe Y, Hayakawa T, Naitoh Y. Colour Doppler-guided spectral analysis of gall-bladder wall flow. J Gastroenterol Hepatol. 1998;13:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Suminski N, Johnson MB, Ralls PW. Color Doppler sonography in gallbladder carcinoma. J Clin Ultrasound. 1991;19:183-186. [PubMed] [DOI] [Full Text] |
| 9. | Li D, Dong BW, Wu YL, Yan K. Image-directed and color Doppler studies of gallbladder tumors. J Clin Ultrasound. 1994;22:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Warren BL. Small vessel occlusion in acute acalculous cholecystitis. Surgery. 1992;111:163-168. [PubMed] |
| 11. | Schiller VL, Turner RR, Sarti DA. Color doppler imaging of the gallbladder wall in acute cholecystitis: sonographic-pathologic correlation. Abdom Imaging. 1996;21:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Mitake M, Nakazawa S, Naitoh Y, Kimoto E, Tsukamoto Y, Yamao K, Inui K. Value of endoscopic ultrasonography in the detection of anomalous connections of the pancreatobiliary duct. Endoscopy. 1991;23:117-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Tanno S, Obara T, Maguchi H, Fujii T, Mizukami Y, Shudo R, Takahashi K, Nishino N, Arisato S, Ura H. Association between anomalous pancreaticobiliary ductal union and adenomyomatosis of the gall-bladder. J Gastroenterol Hepatol. 1998;13:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Chang LY, Wang HP, Wu MS, Huang HT, Wang HH, Lin CC, Lin JT. Anomalous pancreaticobiliary ductal union--an etiologic association of gallbladder cancer and adenomyomatosis. Hepatogastroenterology. 1998;45:2016-2019. [PubMed] |
| 15. | Tanno S, Obara T, Fujii T, Mizukami Y, Shudo R, Nishino N, Ura H, Klein-Szanto AJ, Kohgo Y. Proliferative potential and K-ras mutation in epithelial hyperplasia of the gallbladder in patients with anomalous pancreaticobiliary ductal union. Cancer. 1998;83:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Kaneko K, Ando H, Ito T, Kasai K, Watanabe Y, Seo T. Increased cell proliferation and transforming growth factor-alpha (TGF alpha) in the gall-bladder epithelium of patients with pancreaticobiliary maljunction. Pathol Int. 1996;46:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |