Published online Feb 21, 2005. doi: 10.3748/wjg.v11.i7.1011
Revised: October 2, 2004
Accepted: October 26, 2004
Published online: February 21, 2005
AIM: To observe how acupuncture stimulation influences the visceral nociception in rat and to clarify the interactions between acupuncture or somatic input and visceral nociceptive inputs in the spinal dorsal horn. These will provide scientific base for illustrating the mechanism of acupuncture on visceral pain.
METHODS: Experiments were performed on Sprague-Dawley rats and the visceral nociceptive stimulus was generated by colorectal distention (CRD). Unit discharges from individual single neuron were recorded extracellularly with glass-microelectrode in L1-3 spinal dorsal horn. Acupuncture stimulation was applied at contralateral heterotopic acupoint and ipsilateral homotopic acupoint, both of which were innervated by the same segments that innervate also the colorectal-gut.
RESULTS: The visceral nociception could be inhibited at the spinal level by the heterotopic somatic mechanical stimulation and acupuncture. The maximal inhibition was induced by acupuncture or the somatic noxious stimulation at spinal dorsal horn level with inhibiting rate of 68.61% and 60.79%, respectively (P<0.01 and <0.001). In reversible spinalized rats (cervical-thoracic cold block) both spontaneous activity and responses to CRD increased significantly in 16/20 units examined, indicating the existence of tonic descending inhibition. The inhibition of acupuncture on the noxious CRD disappeared totally in the reversible spinalized rats (P<0.001).
CONCLUSION: The inputs of noxious CRD and acupuncture may interact at the spinal level. The nociceptive visceral inputs could be inhibited by acupuncture applied to hetero-topic acupoint. The effect indicates that the spinal dorsal horn plays a significant role in mediating the inhibition of acupuncture and somatic stimulation on the neuronal response to the noxious visceral stimulation and the inhibition is modulated by upper cervical cord and/or supra-spinal center.
- Citation: Rong PJ, Zhu B, Huang QF, Gao XY, Ben H, Li YH. Acupuncture inhibition on neuronal activity of spinal dorsal horn induced by noxious colorectal distention in rat. World J Gastroenterol 2005; 11(7): 1011-1017
- URL: https://www.wjgnet.com/1007-9327/full/v11/i7/1011.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i7.1011
Visceral pain is poorly understood compared with cutaneous pain, as a consequence, much of our understanding of visceral pain is limited to anecdotally clinical descriptions of pain related to visceral pathology. Abdominal pain associated with stimulation of the gastrointestinal tract has been related to sustained or repetitive distention of the gut, as occurs pathologically with intestinal obstruction. Psychophysical studies have demonstrated that experimental balloon distention of the sigmoid colon also leads to the sensation of pain, but in some cases, only after multiple presentations of the distending stimulus[1,2]. This stimulus in humans also produces sensitization of neighboring structures that are not being directly stimulated, suggesting the activation of central mechanisms[2,3]. Recently, the studies of visceral nociception have employed the stimulus of colorectal distention (CRD) to evoke vigorous physiological, neuronal, and behavioral responses in rats, rabbits, horses, cats, dogs, and primates (including humans)[4]. These responses have been interpreted as painful or nociceptive.
Based on the new development in the concept of central pathways for visceral nociception, recent studies were proposed to investigate the interactions between somatic inputs and nociceptive colorectal inputs in the rat dorsal horn of spinal cord[5]. According to the pattern theory of pain, which assumes that the neuronal discharge rate is related to the level of nociception, the reduced discharge of these nociceptive neurons in response to CRD in the presence of tactile inputs may mean an alleviating effect for visceral pain, and on the other hand, the abnormally increased response to a tactile stimulus of skin may mean skin allodynia or pain referred to the body surface. Furthermore, it is not clear whether the possible sensitive sites on the body surface in association with the visceral pain are related to the acupoints, the Ah-shi acupoint in particular.
Despite rapid development in pharmacology in the last 60 years, only a small proportion of medical care worldwide is delivered by means of conventional, biomedically oriented “western medicine”. An estimated 70% of human healthcare is achieved by means of alternative therapies. The survival of these traditional medicines indicates that they have their merits for being economic, effective, and/or with less side-effects. However, many traditional therapies remain to be called alternative or complementary medicine because they lack modern scientific support.
Being a kind of traditional technique with a long history, acupuncture has been used to treat a variety of diseases including pain. This striking and convincing effect activated the curiosities of scientists and then ample evidence was achieved on its mechanism. Electrophysiological studies show that acupuncture might inhibit the neuronal discharges induced by pain of both somatic and visceral sources in different levels of the central nervous system. This gave a good explanation for the clinic phenomena that in some circumstances acupuncture produces quick effect on pain and relatively slow and long post effect in the other. Though there was vast data relating the depression of somatic afferent inputs on visceral nociception, the evidence about the role of acupuncture in this process did not abound.
In our recent study, we observed that somatic inputs and noxious visceral signals (i.e., CRD) might converge and interact in the neurons at ventroposterior, lateral and dorsal column nucleus levels. Their interactions were manifested as that when the cutaneous stimuli were applied, the neuronal response to CRD was reduced in most cases[6,7].
The current project endeavors to unveil central pain mechanisms in general and visceral nociception in particular, and to shine light on the scientific foundation of “alternative medicine” including acupuncture and touch therapies. By means of electrophysiological recording in the thalamus, we expect to strengthen our preliminary findings that tactile input may suppress nociceptive visceral inputs by virtue of somatovisceral convergence[6], thus providing a scientific basis for somatic therapies. On the other hand, visceral nociception may sometimes cause abnormally higher discharges of thalamic neurons in response to tactile stimulation and hence presumably, skin allodynia or pain referred to the body surface[8]. Acupuncture may be an appropriate method for pain relief in this latter circumstance as shown in many studies[9,10].
The purpose of this experiment was to observe how acupuncture stimulation influences the visceral nociception in rat and to clarify the interactions between somatic and visceral nociceptive inputs in the spinal dorsal horn.
All the animal experiments in the present study were approved authoritatively in accordance with the “Guide for Care and Use of Laboratory Animals” issued by National Institutes of Health. Experiments were performed on 67 Sprague-Dawley rats weighing between 250 and 350 g. The animals were housed with ad libitum access to food and water in a room illuminated from 07.00 to 18.00. The rats were initially anesthetized with an intraperitoneal injection of pentobarbital sodium (50-60 mg/kg). The trachea was intubated and a catheter was inserted into one of the jugular veins to allow a continuous infusion of the anesthetic (maintained with intravenous infusion at 5 mg/(kg·h)). A laminectomy was performed between lumbar 1-3 segments of the spinal cord and the corresponding vertebrae mounted on a rigid frame. Finally, the skin overlying the cord was retracted to form a pool. In reversible spinalization experiments, cervix 8 and thorax 1 laminectomy was also performed to expose the spinal cord for the application of frozen normal saline to produce a reversible cold block.
Discharges from single spinal neurons were recorded extracellularly with the glass microelectrode filled with a solution of NaCl and pontamine sky blue (resistance 9-15 MΩ) and the cord covered with agar gel to minimize the movements caused by respiration. The isolated action potentials were fed into a window discriminator and displayed on an oscilloscope screen. The output of the window discriminator and amplifier were led into a data collection system and a personal computer data acquisition system (PowerLab) for further analysis.
The excitatory receptive field of each neuron was mapped first by innocuous tactile stimulation and then its response to CRD with pressure ranging from 20 to 100 mmHg was tested. Only those neurons that responded to both cutaneous stimuli and CRD were recruited in the present study. Furthermore, the neurons selected for the present study were actually the convergent units, which responded to both noxious (strong pinch and noxiously hot water) and innocuous (hair movement, pressure) stimuli applied to the receptive field. Correspondingly, they could also be activated by both large A- and fine C-fibers during transcutaneous electrical stimulation delivered through a pair of stainless steel needles inserted in the center of the excitatory receptive field of the neurons.
The unit discharges induced by the stimulation were compared with the background activity recorded before any stimulation was added (“net response”). If the impulse counts during stimulation were 20% more or less than the background activity, the neuron was then considered to have an excitatory or inhibitory response respectively.
Heart rate was continuously monitored and body temperature kept constantly around 37 °C by means of a feedback-controlled heating blanket.
The noxious visceral stimulus employed in these studies consisted of distention of the descending colon and rectum of the rat by air inflation of a 4 cm flexible latex balloon. The balloon was inserted via the anus and kept in position (the end of the balloon was 1 cm from the anus) by tying the connecting catheter to the tail of the animal. Pressure within the balloon was controlled using a blood-pressure meter and increased repeatedly to 80-100 mmHg for 50 s with an interval of 5 min to avoid overstimulation and sensitization. CRD stimuli with intensity >40 mmHg are recognized as noxious based on previous studies and experience. CRD has been extensively used as a visceral pain model since it produces pain in humans and evokes avoidance behavior in rats[11].
Excitatory somatic receptive fields were mapped. Neurons were classified by their responses to the innocuous (brush and tap) and noxious (pinch) stimuli. The skin receptive field was identified initially by gentle tapping and brushing, and then defined with von Frey hairs.
Manual acupuncture was applied at the excitatory receptive field of ipsilateral hindlimb acupoint St-36 (Zusanli, located below the capitulum fibulae and lateral to the tibia) and non-receptive field, contralateral hindlimb acupoint St-36. For the manual acupuncture, a needle was inserted into acupoint and was rotated clockwise and anti-clockwise at 2 Hz for 30 s.
When cell activity is well isolated from background noise, the response of the neuron to skin stimulation and CRD will be tested separately first. The skin receptive field will be mapped by means of von Frey hairs. Visceral nociceptive stimulation will be generated by CRD by means of a balloon catheter inserted into the descending colon and rectum with a pressure of up to 80-100 mmHg.
A standard protocol was employed for the determination of effects of acupuncture and somatic stimuli on the neuronal response. A background activity of the neurons was recorded for 5-20 s followed by a test of their responses to CRD stimulation for 50 s. Manual acupuncture at the acupoint or mechanical stimulation on the skin was applied for 30 s beginning at the tenth second of a 50-s period of CRD stimulation. After the acupuncture or somatic stimulation, the response of the neurons was recorded for another 10 s and finally, the background activity of the neurons was tested for another 5 s after the CRD stimulation was removed.
A lesion was made at the site of recording or at the end of an electrode track by passing 100- to 500-μA DC current for 10-30 s to identify the locations of neurons recorded in the spinal cord. The fixed brain was blocked and sectioned at a thickness of 50-μm and the locations of recording site within the dorsal horn were verified from specimens by reconstruction of microelectrode tracks.
The data obtained before and after intervention in the same group used paired t-test, and the corresponding ones between the two groups were compared statistically by an independent t-test. P<0.05 was considered as statistical significance. All data are presented as mean±SE.
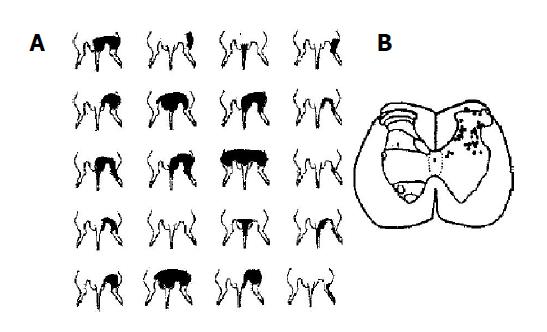
A total of 237 spinal dorsal horn neurons in L1-3 segments were recorded in 67 rats, of which, 145 were convergent neurons, 80 were those responding only to innocuous stimulation (non-noxious-only neurons) and 12 were the ones responding only to the noxious stimulation (noxious-only neurons). As shown by electrophoresis of the dye at the end of experiments, the convergent neurons in the present study were located within the dorsal horn both in superficial (lamina 1) and, more often, in deeper zones (laminae 4-6) of the gray matter (Figure 1B), while their excitatory receptive fields were located in the caudal parts of the body, including the scrotum, hip region, thigh, hindlimb, or tail, and were sometimes even larger (Figure 1A). The neuron could be activated by both noxious (pinch, hot water) and innocuous (von Frey hairs, tapping, light pressure) stimuli; the application of sustained non-noxious pressure resulted in a phasic response prior to a short period of adaptation, which was followed by a tonic discharge lasting throughout the period of stimulation. By applying 1 ms duration transcutaneous electrical square-wave stimuli to the center of their excitatory receptive fields, responses due to peripheral activation of A- and C-fibers could be observed in application of suprathreshold current.
Eighty non-noxious-only neurons had a distal cutaneous excitatory receptive field; they responded only to innocuous peripheral stimuli such as hair movement, light pressure and touch, but did not respond to noxious stimuli, or to C-fiber inputs. The non-noxious-only neurons were found in laminae 3-6. Twelve noxious-only units were mainly located in the marginal layer of the dorsal horn. The peripheral receptive fields were smaller than those of the convergent neurons. The neurons could only be activated by a strong pinch or noxious heat and received a clear C-fiber input without A-fiber input.
In the present study, we focused on the convergent neuron, i.e., the units responding to both noxious and non-noxious inputs.
All convergent neurons were excited by noxious and non-noxious cutaneous stimuli, and were also activated by CRD stimuli. Among 145 units responding to CRD, 113 were excited and 32 were inhibited. In general, the neurons were classified as abrupt or sustained neurons[10]. The abrupt neurons were excited with short latency by CRD and their activities abruptly returned to the baseline or under baseline levels after the termination of distending stimulus. The sustained neurons were also excited with short latency by CRD, but the responses were sustained for longer than 5 s after termination of distention. Whereas, the activity of both abrupt and sustained neurons was similarly attenuated by acupuncture stimulation applied at non-segmental acupoints.
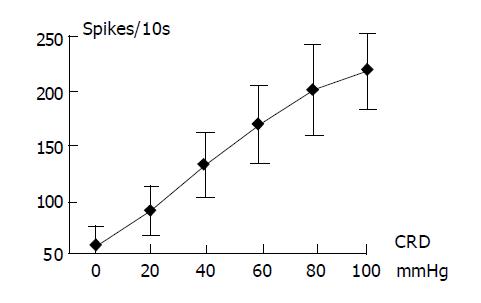
As shown in Figure 2, the convergent neurons responded linearly to the CRD of increasing intensities from 20 to 100 mmHg. During CRD at 100 mmHg, these neuronal discharges increased from 5.95±1.87 spikes/s, the baseline level, to 21.67±3.41 spikes/s, with an increasing rate of 364.20%.
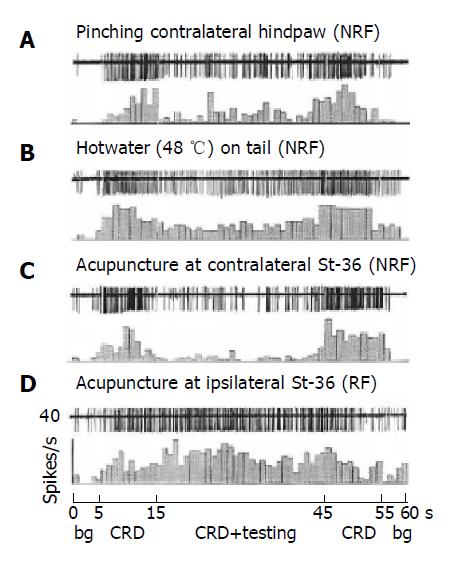
The effect of noxiously conditioning pinch of skin of non-receptive field on neuronal responses to CRD was studied in 18 convergent neurons. As an individual example shown in Figure 3, the firing of the neuron was induced by sustained CRD at 100 mmHg, it was shown that either noxious pinch of hind paw (Figure 3A and Figure 4) or the stimulation of tail by hot water at 48 °C (Figure 3B) strongly reduced the responses of the neurons to CRD, whereas non-noxious light pressure was totally ineffective.
The noxious pinch of homotopic region i.e., the somatic region converged with visceral inputs in excitatory receptive field of convergent units induced a significant facilitation of the response to CRD (Figure 4).
The effect of acupuncture of non-receptive field (usually at contralateral St-36 acupoint) on CRD-evoked response was also investigated in the present study. As shown in Figure 3C for a representative individual example, CRD-evoked response of convergent unit could be strongly inhibited by heterotopic acupuncture at contralateral St-36 acupoint (Figure 3C). However, acupuncture stimulation of excitatory receptive field (usually at ipsilateral St-36 acupoint) produced a strongly facilitative effect on the response of the neuron to CRD (Figure 3D).
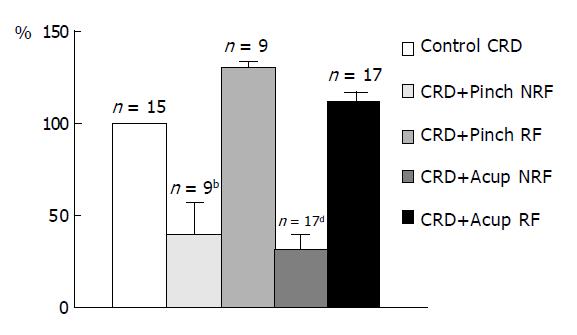
The results of acupuncture and mechanical stimulations on the responses of convergent neurons to CRD were summarized in Figure 4. Both heterotopic noxious mechanical stimuli and heterotopic acupuncture could induce a similar inhibitory effect on the responses of convergent neurons to CRD while, either noxious mechanical stimuli or acupuncture at the neuronal excitatory receptive field significantly facilitated the responses of the neurons to CRD.
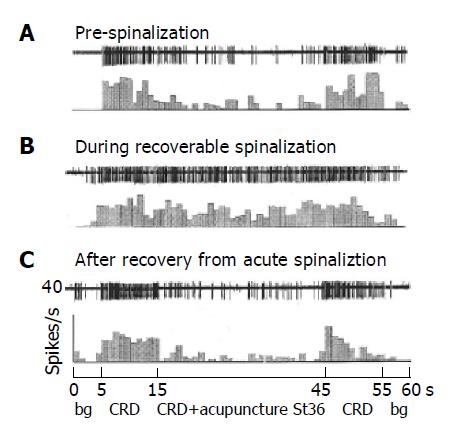
Twenty convergent neurons were recorded in 14 reversible spinalization rats. All the 20 neurons in the rats were found to respond to the noxious heat, strong pinch, pressure and light touch and received A and C fiber inputs before spinalization. After spinalization, the spontaneous discharges (background activity) of the neurons were 5.7±1.1 spikes/s, which was significantly enhanced as compared to 3.9±0.9 spikes/s, obtained before spinalization (P<0.05). Similarly, the responses of the neurons to sustained CRD at 80 mmHg were 14.9±2.5 spikes/s following spinalization, which was statistically higher than that of pre-spinalization, 12.7±3.5 spikes/s (P<0.05).
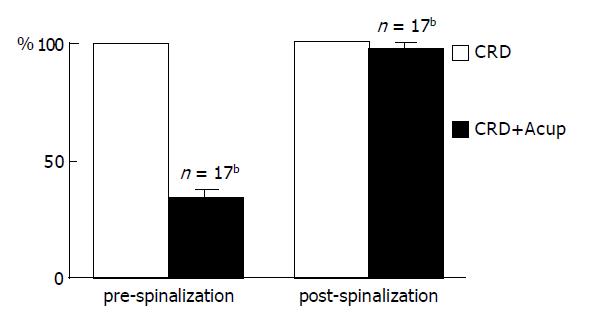
As shown in Figure 5, the responses of 20 convergent neurons to sustained CRD were significantly inhibited by acupuncture at contralateral St-36 acupoint with an inhibitory rate of 66.7±3.5% in the pre-spinalized rats (Figure 5A). Interestingly, the inhibitory effect of the same stimulation of acupuncture almost completely disappeared in the same rats after spinalization (Figure 5B). The inhibitory effect could reoccur in several minutes after the spinal blockage was removed (Figure 5C and Figure 6 for summary data).
Over the last few decades there has been a widespread and increasing interest in acupuncture around the world. Though great progresses are made, a lot of mysteries and disputes still remain in acupuncture mechanism. It was confirmed that acupuncture could produce obvious inhibition on pain-related discharges of the neurons in almost all levels of the central nervous system. Acupuncture-induced analgesia is widely accepted in the fields of both clinical and basic science studies[9,12]. As mentioned in the section of introduction, there is the inter-inhibitory control at the spinal level that converge and integrate the information coming from both somatic (including acupuncture-activated input) and visceral afferents. In the present study, acupuncture or pinch stimulation applied in the area remote to the receptive field induced a rapid suppression on the response to the noxious visceral stimulation. Actually, the conditioning stimulation of various areas of the entire body could produce the inhibition in an intensity-dependent manner. These features resemble diffuse noxious inhibitory control (DNIC) phenomena[13].
Quantitative neurophysiological studies in rats have demonstrated the existence of at least two spinal neuron populations that encode for CRD in an excitatory, graded fashion throughout the noxious range[14,15]. The recent study conducted by Ness and Gebhart[16] demonstrated that chemical inflammation of the colon-rectum leads to increases in reflex responses to CRD and to increased CRD-evoked activity in a subset of spinal dorsal horn neurons encoding for CRD, the sustained neurons. The magnitude of the alterations in the reflex responses correlated in time with an index of local inflammation, the extravasation of Evans blue into colorectal tissues. The mechanisms of these increased responses may be due to both processes at the level of the primary afferent transducer as well as central processes (spinal cord or brain).
In terms of nociception, the spinothalamic tract (STT) has been viewed as the most important pathway for nociception, including visceral pain, and previous studies of pain mechanisms have focused mainly on this pathway and its related projection areas[17,18]. There has been ample evidence to show that viscerosomatic convergence is a common phenomenon in this pathway[19,20], and it has been found that virtually all second-order neurons in the spinal cord that receive visceral inputs also have convergent somatic inputs[21,22]. Studies of pain mechanisms and viscerosomatic interactions have also been mainly aimed at the spinal level. The interactions between different fibers have been conducted in the STT and it is generally found that the interaction of the two afferent systems is usually an inhibition of the second response irrespective of which of the systems is activated first[19,20,23], and inhibition of one nociceptive response is better achieved with another painful conditioning stimulus. As most STT neurons respond specifically to noxious inputs or have wide dynamic range responses[21], studies of pain control by the conditioning method have so far invoked either electrical[15,16] or nociceptive stimuli[19,20]. Despite an early report in which electrical stimulation that was considered to activate large diameter fibers (and thus was innocuous) could temporarily abolish pain in human subjects[26], activation of Aδ and C fibers in general was found necessary in order to efficiently inhibit or counteri-rritate the original pain[27,28]. In principle, the electrical nerve stimulation involves “nocigenic inhibition” or “counteri-rritation”, in line with the DNIC mechanism[29].
Numerous studies have shown that viscerosomatic convergence appears to be a rule rather than an exception at both the spinal and supraspinal levels[30]. The spinal convergent neurons receive signals from viscera, muscles and joints. This convergence of inputs means that multi-receptive neurons are continuously capturing all the information from both the external environment (the skin) and the internal milieu (the viscera, muscles, etc.). This information constitutes a ‘basic somaesthetic activity’ that could help the somatosensory system to build a ‘global representation of the body’. In addition to a global entity, the output of multi-receptive neurons should be understood in dynamic terms since the size of the peripheral fields of the individual neurons may change, as a result of the plasticity of both excitatory and inhibitory segmental processes. Furthermore, the activity of these neurons can be inhibited from most of the remaining parts of the body via supraspinal mechanisms. These DNICs are triggered by peripheral Aδ- and C-fibres, involving the brain structures confined to the caudal-most part of the medulla[29]. We previously reported that acupuncture stimulation might inhibit C- nociceptive inputs produced by electrical shock in the excitatory receptive fields of the convergent neurons in spinal and bulb dorsal horn. The inhibition was strong and lasted for long period[31]. The present data not only support the findings of earlier reports of nociceptive inhibition in cutaneous models, but also extend them to visceral system. Ness and Gebhart’s studies[19,20] on nociceptive inhibition of neuronal and/or reflex responses to a reproducible, reliable, noxious visceral stimulation (for CRD), showed that the noxious conditioning cutaneous stimuli inhibited the responses to the gradually-increased noxiously visceral stimulus in an intensity-dependent fashion and made a parallel shift to the right of stimulus-response curve. In human, Coffin and colleagues observed that transcutaneous electrical nerve stimulation (100 Hz, 100 μs) could reduce the perception of gut distention[32]. Meanwhile, electrical stimulation on the dorsal surface of spinal cord at L1 could obviously attenuate the behavioral response of visceromotor induced by CRD (60 mmHg for 10 min) in a rat model. The authors suggested that, like somatic stimulation, the spinal cord stimulation might have therapeutic potential for the treatment of visceral pain[33].
The current study showed that the skin areas that may interact with colorectal nociception at the spinal[10,11] and thalamic[34] levels are located in the caudal part of the body including the tail, hindlimb, perineal and hip regions. In terms of the spinal segmental arrangement, they are within the lumbosacral dermatomes, the same segments that innervate the hindgut (L2-S3). Despite having generally smaller receptive fields on the extremities than those on the abdominal region, there was no clear correlation between the type of interactions, whether excitatory or inhibitory, and the skin receptive fields from our limited data. Whether the skin spots, in particular the tender points, where the interactions take place are related to acupoints and the meridians of traditional Chinese medicine remains to be investigated further, preferably in primates.
It is generally accepted that multiple supraspinal sites of the descending pain modulatory system exert powerful effects on the inhibitory response of the nociceptive messages at the spinal level. It has been demonstrated that acupuncture stimulation inhibited Fos expression in the dorsal horn induced by mechanical noxious stimulation or hindlimb amputation and inhibited the nociceptive response[35]. The results here in reversible spinalized experiments also indicated that the neuronal inhibition on noxious CRD by acupuncture totally disappeared. However, Ness and Gebhart[10] certified that noxious conditioning cutaneous stimuli could still inhibit CRD-induced neuronal responses in the spinal cord transection at the level of C1 segment; this result was not in coincidence with the present observation. Actually, the propriospinal neurons in the upper cervical cord (C1-C3) can receive a convergence from the different visceral inputs and serve as a potential integrator of sensory information between visceral organs separated by distance and function. This multisegmental modulation of visceral afferent processing still occurs after C1 transection but is abolished after a C6 transection[36-38]. These researches indicate that the neurons of upper cervical segments may be important for modulatory activity of distant spinal neurons responding to visceral input (processor). Recent studies have suggested that electroacupuncture-activated spinal neurons convey acupuncture signals to the brain and activate a descending inhibitory system, which in turn inhibits c-Fos expression in the spinal cord and hyperalgesia[39,40].
Based upon the finding of the present and previous studies, it can be concluded that the convergent neurons of spinal dorsal horn is an important area of visceral nociceptive processing. These findings further extend and support the contention that visceral nociception occurs in the lower lumbar spinal cord of the rat. The neurons respond to the gradually-increased intensity of noxious visceral stimulation in an intensity-dependent manner. Noxious conditioning cutaneous stimuli and acupuncture at acupoint can significantly inhibit the noxiously visceral response in intact, but not in spinalized rats, suggesting that upper cervical cord and/or supraspinal mechanisms are/is important in mediating the nociceptive inhibition produced by the conditioning cutaneous stimuli or acupuncture.
This work supported by grants from China Foundation of Nature Science (30100245). We gratefully acknowledge Professor Yu XC for help in preparing this manuscript.
| 1. | Mayer EE, Munakata J, Mertz H, Lembo T, Bernstein CN. Visceral hyperalgesia and irritable bowel syndrome. Seattle: IASP 1995; 429-467. |
| 2. | Ness TJ, Metcalf AM, Gebhart GF. A psychophysiological study in humans using phasic colonic distension as a noxious visceral stimulus. Pain. 1990;43:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 167] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Munakata J, Naliboff B, Harraf F, Kodner A, Lembo T, Chang L, Silverman DH, Mayer EA. Repetitive sigmoid stimulation induces rectal hyperalgesia in patients with irritable bowel syndrome. Gastroenterology. 1997;112:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 253] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Ness TJ. Models of Visceral Nociception. ILAR J. 1999;40:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Ness TJ, Gebhart GF. Characterization of neuronal responses to noxious visceral and somatic stimuli in the medial lumbosacral spinal cord of the rat. J Neurophysiol. 1987;57:1867-1892. [PubMed] |
| 6. | Zhang HQ, Al-Chaer ED, Willis WD. Effect of tactile inputs on thalamic responses to noxious colorectal distension in rat. J Neurophysiol. 2002;88:1185-1196. [PubMed] |
| 7. | Rong PJ, Zhang JL, Zhang HQ. Interactions between tactile and noxious visceral inputs in rat nucleus gracilus. Neurosci Lett. 2004;362:162-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Zhang HQ, Rong PJ, Zhang SP, Al-Chaer ED, Willis WD. Noxious visceral inputs enhance cutaneous tactile response in rat thalamus. Neurosci Lett. 2003;336:109-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003;26:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 680] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 10. | Ulett GA, Han S, Han JS. Electroacupuncture: mechanisms and clinical application. Biol Psychiatry. 1998;44:129-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 467] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 11. | Ness TJ, Piper JG, Follett KA. The effect of spinal analgesia on visceral nociceptive neurons in caudal medulla of the rat. Anesth Analg. 1999;89:721-726. [PubMed] |
| 12. | Mayer DJ. Biological mechanisms of acupuncture. Prog Brain Res. 2000;122:457-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Murase K, Kawakita K. Diffuse noxious inhibitory controls in anti-nociception produced by acupuncture and moxibustion on trigeminal caudalis neurons in rats. Jpn J Physiol. 2000;50:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Ness TJ, Gebhart GF. Characterization of neuronal responses to noxious visceral and somatic stimuli in the medial lumbosacral spinal cord of the rat. J Neurophysiol. 1987;57:1867-1892. |
| 15. | Ness TJ, Gebhart GF. Characterization of neurons responsive to noxious colorectal distension in the T13-L2 spinal cord of the rat. J Neurophysiol. 1988;60:1419-1438. [PubMed] |
| 16. | Ness TJ, Gebhart GF. Inflammation enhances reflex and spinal neuron responses to noxious visceral stimulation in rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G649-G657. [PubMed] |
| 17. | Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1220] [Cited by in RCA: 1172] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 18. | Willis WD. The pain system. The neural basis of nociceptive transmission in the mammalian nervous system. Pain Headache. 1985;8:1-346. [PubMed] |
| 19. | Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nociception in the rat. I. Noxious cutaneous stimuli inhibit visceral nociceptive neurons and reflexes. J Neurophysiol. 1991;66:20-28. [PubMed] |
| 20. | Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nociception in the rat. II. Noxious visceral stimuli inhibit cutaneous nociceptive neurons and reflexes. J Neurophysiol. 1991;66:29-39. [PubMed] |
| 21. | Foreman RD, Blair RW, Weber RN. Viscerosomatic convergence onto T2-T4 spinoreticular, spinoreticular-spinothalamic, and spinothalamic tract neurons in the cat. Exp Neurol. 1984;85:597-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Gebhart GF, Ness TJ. Central mechanisms of visceral pain. Can J Physiol Pharmacol. 1991;69:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Brennan TJ, Oh UT, Hobbs SF, Garrison DW, Foreman RD. Urinary bladder and hindlimb afferent input inhibits activity of primate T2-T5 spinothalamic tract neurons. J Neurophysiol. 1989;61:573-588. [PubMed] |
| 24. | Foreman RD, Hancock MB, Willis WD. Responses of spinothalamic tract cells in the thoracic spinal cord of the monkey to cutaneous and visceral inputs. Pain. 1981;11:149-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Chung JM, Fang ZR, Hori Y, Lee KH, Willis WD. Prolonged inhibition of primate spinothalamic tract cells by peripheral nerve stimulation. Pain. 1984;19:259-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 104] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Wall PD, Sweet WH. Temporary abolition of pain in man. Science. 1967;155:108-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 696] [Cited by in RCA: 592] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 27. | Chung JM, Lee KH, Hori Y, Endo K, Willis WD. Factors influencing peripheral nerve stimulation produced inhibition of primate spinothalamic tract cells. Pain. 1984;19:277-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 135] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Gerhart KD, Yezierski RP, Giesler GJ, Willis WD. Inhibitory receptive fields of primate spinothalamic tract cells. J Neurophysiol. 1981;46:1309-1325. [PubMed] |
| 29. | Le Bars D. The whole body receptive field of dorsal horn multireceptive neurones. Brain Res Brain Res Rev. 2002;40:29-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 301] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 30. | Ness TJ, Gebhart GF. Visceral pain: a review of experimental studies. Pain. 1990;41:167-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 423] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 31. | Bing Z, Villanueva L, Le Bars D. Acupuncture and diffuse noxious inhibitory controls: naloxone-reversible depression of activities of trigeminal convergent neurons. Neuroscience. 1990;37:809-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 104] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Coffin B, Azpiroz F, Malagelada JR. Somatic stimulation reduces perception of gut distention in humans. Gastroenterology. 1994;107:1636-1642. [PubMed] |
| 33. | Greenwood-Van Meerveld B, Johnson AC, Foreman RD, Linderoth B. Attenuation by spinal cord stimulation of a nociceptive reflex generated by colorectal distention in a rat model. Auton Neurosci. 2003;104:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Takahashi S, Suzuki M, Matsumoto K, Ishii K, Higano S, Fukasawa H, Sakamoto K. Extent and location of cerebral infarcts on multiplanar MR images: correlation with distribution of perforating arteries on cerebral angiograms and on cadaveric microangiograms. AJR Am J Roentgenol. 1994;163:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Lee JH, Beitz AJ. Electroacupuncture modifies the expression of c-fos in the spinal cord induced by noxious stimulation. Brain Res. 1992;577:80-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 67] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Qin C, Chandler MJ, Miller KE, Foreman RD. Responses and afferent pathways of C(1)-C(2) spinal neurons to gastric distension in rats. Auton Neurosci. 2003;104:128-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Chandler MJ, Zhang J, Qin C, Foreman RD. Spinal inhibitory effects of cardiopulmonary afferent inputs in monkeys: neuronal processing in high cervical segments. J Neurophysiol. 2002;87:1290-1302. [PubMed] |
| 38. | Chandler MJ, Qin C, Zhang J, Foreman RD. Differential effects of urinary bladder distension on high cervical projection neurons in primates. Brain Res. 2002;949:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Lao L, Zhang G, Wei F, Berman BM, Ren K. Electro-acupuncture attenuates behavioral hyperalgesia and selectively reduces spinal Fos protein expression in rats with persistent inflammation. J Pain. 2001;2:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS). J Pharmacol Exp Ther. 2001;298:257-263. [PubMed] |