Published online Feb 14, 2005. doi: 10.3748/wjg.v11.i6.858
Revised: May 9, 2004
Accepted: July 6, 2004
Published online: February 14, 2005
AIM: To investigate the possible association of G→A single nucleotide polymorphism (SNP) at the -1082 position of interleukin (IL)-10 promoter with susceptibility to esophageal squamous cell carcinoma (ESCC) and gastric cardiac adenocarcinoma (GCA) in a population of a high incidence region of North China.
METHODS: IL-10-G1082A promoter SNP was genotyped by polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP) in 355 cancer patients (203 ESCC and 152 GCA) and 443 healthy controls.
RESULTS: Smoking significantly increased the risk of ESCC and GCA development (the age and sex adjusted OR = 1.42 and 2.64, 95%CI = 1.11-1.81 and 1.46-4.76, respectively). Similarly, family history of upper gastrointestinal cancer (UGIC) significantly increased the risk of developing ESCC and GCA (the age and sex adjusted OR = 1.44 and 3.10, 95%CI = 1.18-1.75 and 1.94-4.97, respectively). The A/A, A/G and G/G genotype frequencies of IL-10-G1082A were 60.3%, 37.0% and 2.7% in healthy controls, 57.6%, 39.9% and 2.5% in ESCC and 61.2%, 36.8% and 2.0% in GCA patients, respectively. The frequencies of A and G alleles were 78.8% and 21.2% in healthy controls, 77.6% and 22.4% in ESCC patients and 79.6%, 20.4% in GCA patients. The distribution of genotype and allelotype in ESCC and GCA patients was not significantly different from that in healthy controls (P>0.05). Compared to the A/A genotype, the combination of A/G and G/G genotypes did not show a significant effect on the risk of developing ESCC and GCA; the adjusted odds ratio was 0.92 (95% CI = 0.76-1.11) in ESCC and 0.95 (95% CI = 0.61-1.46) in GCA, respectively. When stratified for smoking status and family history of UGIC, the combination of A/G and G/G genotypes also did not show any significant influence on the risk of ESCC and GCA development compared to A/A genotypes.
CONCLUSION: IL-10-G1082A polymorphism might not be used as a stratification marker to predicate the risk of ESCC and GCA development in North China.
- Citation: Guo W, Wang N, Wang YM, Li Y, Wen DG, Chen ZF, He YT, Zhang JH. Interleukin-10 -1082 promoter polymorphism is not associated with susceptibility to esophageal squamous cell carcinoma and gastric cardiac adenocarcinoma in a population of high-incidence region of north China. World J Gastroenterol 2005; 11(6): 858-862
- URL: https://www.wjgnet.com/1007-9327/full/v11/i6/858.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i6.858
China is a country with high-incidence regions of esophageal squamous cell carcinoma (ESCC) and gastric cardiac adenocarcinoma (GCA). Exogenous factors including nutrition deficiency[1], unhealthy living habits[2], consumption of alcohol and tobacco[2,3], pathogenic infections[4,5] are generally considered as the risk factors for developing these two cancers in China. However, only a subset of individuals exposed to the above listed exogenous risk factors would develop ESCC and GCA, suggesting a role of host susceptibility factors in cancer development. The role of a genetic background in developing these cancers has also been strongly suggested by familial clustering of upper gastrointestinal cancer (UGIC) patients in high-incidence regions[6,7]. Some candidate genes have been identified to modify the susceptibility to ESCC and GCA in Chinese or other populations[8].
Cytokines play an important role in regulating both humor and cell-mediated immune responses. Promoter regions of some cytokine genes contain polymorphisms that may directly influence the cytokine transcription or expression[9]. These promoter polymorphisms may lead to either high- or low-level production of the given cytokines, cause inter-individual differences in antitumor immune response and subsequently influence the susceptibility to cancers. For example, vascular endothelial growth factor (VEGF) polymorphism has been suggested to affect the susceptibility to prostate cancer by promoting angiogenesis[10]. Interleukin-1 polymorphism has been demonstrated to be a risk factor for gastric cancer in subjects with H pylori infection[11].
Interleukin (IL)-10, which is produced by T-lymphocytes, is an important anti-inflammatory and immuno-suppressive cytokine and may regulate angiogenesis in various cancers. The IL-10 gene is located on chromosome 1q31-32. Polymorphisms at positions -1082, -819 and -592 of the IL-10 promoter region are correlated with IL-10 production[12]. The expression of IL-10 is correlated to angiogenic factor expression in ESCC that may influence the development and progression of this tumor[13]. It has been reported that IL-10-G1082A polymorphism is correlated with the expression of IL-10 and accordingly affects the susceptibility to some types of tumors, such as cervical cancer[14] and prostate cancer[10]. Although the expression of IL-10 gene might be associated with the susceptibility to ESCC[13], the correlation of IL-10 promoter polymorphism with the susceptibility to ESCC and GCA has not been reported so far. Therefore, in this study, we conducted a population-based case control study to investigate the association of IL-10 -G1082A polymorphisms with the risk of ESCC and GCA development in Cixian County and Shexian County, a high-incidence region of ESCC and GCA in Taihang Mountain, North China.
This study included 355 patients (203 with ESCC and 152 with GAC) and 443 healthy individuals without overt cancer. The cases were outpatients for endoscopic biopsy or inpatients for tumor resection in the Fourth Affiliated Hospital of Hebei Medical University or local tumor hospitals in Cixian County and Shexian County between 2001 and 2003. Histological tumor typing was carried out on the basis of biopsy or resection specimens. Esophageal carcinomas were all squamous cell carcinomas. Gastric cardiac carcinomas were all adenocarcinomas with their epicenters at the gastroesophageal junction, i.e., from 1 cm above until 2 cm below the junction between the end of the tubular esophagus and the beginning of the saccular stomach[15]. Healthy subjects were recruited from Cixian County and Shexian County during the endoscopic screening campaign between 2001 and 2003. All the cancer patients and control subjects were unrelated Han nationals. Information of sex, age, smoking habit and family history was obtained from cancer patients and healthy controls by an interview following sampling. Smokers were defined as former or current individuals smoking 5 cigarettes per day for at least two years. Individuals with at least one first-degree relative or at least two second-degree relatives having esophageal/cardiac/gastric cancer were defined as having a family history of upper gastrointestinal cancers (UGIC). Smoking status and family history were only available from a sub-set of cancer patients and healthy controls (Table 1). The study was approved by the Ethics Committee of Hebei Cancer Institute and informed consent was obtained from all recruited subjects.
| Groups | Control, n (%) | ESCC, n (%) | Pa | GCA, n (%) | P1 |
| Sex | |||||
| Male | 269 (60.7) | 135 (66.5) | 0.16 | 102 (67.1) | 0.16 |
| Female | 174 (39.3) | 68 (33.5) | 50 (32.9) | ||
| Mean age, in years (SD) | 57.1 (10.27) | 59.2 (9.58) | 0.062 | 60.7 (8.07) | 0.062 |
| Smoking status3 | |||||
| Ex- or current smoker | 144 (33.2) | 106 (55.5) | <0.0015 | 88 (66.7) | <0.0015 |
| Non-smoker | 290 (66.8) | 85 (44.5) | 44 (33.3) | ||
| Family history of UGIC4 | |||||
| Positive | 164 (37.0) | 93 (52.2) | <0.001f | 74 (61.2) | <0.0016 |
| Negative | 279 (63.0) | 85 (47.8) | 47 (38.8) | ||
| IL-10 -G1082A SNP genotype | |||||
| A/A | 267 (60.3) | 117 (57.6) | 0.78 | 93 (61.2) | 0.88 |
| A/G | 164 (37.0) | 81 (39.9) | 56 (36.8) | ||
| G/G | 12 (2.7) | 5 (2.5) | 30 (2.0) | ||
| IL-10 -G1082A SNP allelotype | |||||
| A | 698 (78.8) | 315 (77.6) | 0.63 | 242 (79.6) | 0.76 |
| G | 188 (21.2) | 91 (22.4) | 62 (20.4) |
Five milliliters of venous blood from each subject was drawn into Vacutainer tubes containing EDTA and stored at 4 °C. Genomic DNA was extracted within one week after sampling by using proteinase K (Merck, Darmstadt, Germany) digestion followed by a salting out procedure according to the method published by Miller[16].
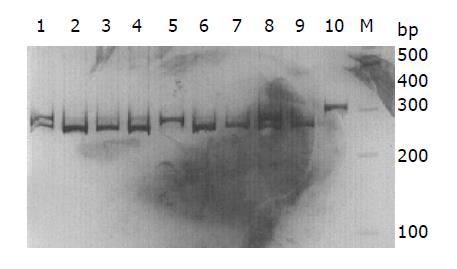
IL-10-G1082A genotyping was determined by polymerase-chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay. PCR primers used for amplifying IL-10-G1082A fragments were 5’-CACTACTAAGGCTTCCTTGGGA-3’ (sense) and 5’-GTGAGCAAACTGAGGCACAGACAT-3’ (antisense). The sense primer was specially designed to introduce a recognition site of restriction enzyme EcoN I (CCTNN\NNNAGG, MBI Fermentas, Lithuania) by replacing a T with a C at the seventh position close to the 3’ end of the primer. PCR was performed in a 20 µL volume containing 100 ng of DNA template, 2.0 µL of 10×PCR buffer, 1.5 mmoL of MgCl2, 1 U of Taq-DNA-polymerase (BioDev-Tech., Beijing, China), 200 µmoL of dNTPs and 200 nmoL of sense and antisense primers. The PCR cycling conditions were at 94 °C for 5 min followed by 35 cycles at 94 °C for 30 s, at 60 °C for 50 s, and at 72 °C for 40 s, and with a final step at 72 °C for 5 min to allow the complete extension of all PCR fragments. An 8 µL aliquot of PCR products was digested overnight at 37 °C in a 10 µL reaction containing 10 units of EcoN I and 1×reaction buffer. After an overnight digestion, the products were resolved and separated on a 10% polyacrylamide gel followed by staining with 0.25% silver nitrate. After electrophoresis, the AA homozygote was represented by three DNA bands being 252, 39 and 19 bp in length. The GG homozygote was represented by two DNA bands being 271 and 39 bp in length, whereas the heterozygote displayed four DNA bands being 271, 252, 39 and 19 bp in length (Figure 1). For a negative control, each PCR reaction used distilled water instead of DNA in the reaction system. For 10% of the samples, the reaction was repeated once for IL-10 genotyping and all of the genotypes matched with the original results.
Statistical analysis was performed using SPSS10.0 software package (SPSS Company, Chicago, IL). Hardy-Weinberg analysis was performed by comparing the observed and expected genotype frequencies using χ2 test. Comparison of the IL-10-G1082A genotype distribution in ESCC and healthy controls was performed by means of two-sided contingency tables using χ2 test. A probability level of 5% was considered statistically significant. The odds ratio (OR) and 95% confidence interval (CI) were calculated using an unconditional logistic regression model and adjusted by age and sex accordingly.
The mean age of ESCC cases, GCA cases and controls was 59.2±9.58 years (range 34-85), 60.7±8.07 years (range 37-86) and 57.1±10.27 years (range 31-78), respectively. The gender distribution in ESCC and GCA patients (66.5% and 67.1% men) was comparable to that in healthy controls (60.7% men) (P = 0.16 and 0.16, respectively). The proportion of smokers in ESCC and GCA patients (55.5% and 66.7%) was significantly higher than that in healthy controls (33.2%) (χ2 = 27.53 and 46.92, respectively, P<0.001). Therefore, smoking significantly increased the risk of ESCC and GCA development (the age and sex adjusted OR = 1.42 and 2.64, 95% CI = 1.11-1.81 and 1.46-4.76, respectively). In addition, the frequency of positive family history of UGIC in ESCC (52.2%) and GCA (61.2%) patients was significantly higher than that in healthy controls (37.0%) (χ2 = 12.14 and 22.70, respectively, P<0.001). Thus, family history of UGIC significantly increased the risk of developing ESCC (adjusted OR = 1.44, 95%CI = 1.18-1.75) and GCA (adjusted OR = 3.10, 95%CI = 1.94-4.97). The demographic distribution of ESCC and GCA patients as well as healthy controls was shown in Table 1.
IL-10 genotyping was successfully performed in all study subjects. The genotype distribution in ESCC and GCA patients and healthy controls was consistent with Hardy-Weinberg equilibrium (P = 0.30, 0.47 and 0.23, respectively). The genotype distribution was not correlated with gender and age both in healthy controls and in ESCC and GCA patients (data not shown). In healthy controls, the frequencies of the A/A, A/G and G/G genotypes were 60.3%, 37.0% and 2.7%, while the distribution of A and G alleles was 78.8% and 21.2%, respectively. As shown in Table 1, there was no statistical difference in allele distribution between ESCC, GCA patients and healthy controls (χ2 = 0.24 and 0.09, P = 0.63 and 0.76, respectively). The overall IL-10 genotype distribution in ESCC and GCA patients was also not significantly different from that in healthy controls (χ2 = 0.50 and 0.26, P = 0.78 and 0.88, respectively). Compared to the A/A genotype, the combination of A/G and G/G genotypes did not show a significant effect on modifying the risk of ESCC and GCA development; the adjusted OR for ESCC and GCA was 0.92 (95%CI = 0.76–1.11) and 0.95 (95% CI = 0.61–1.46), respectively.
When stratified for smoking status and family history of UGIC, the frequency of IL-10 genotypes in ESCC and GCA patients was also not significantly different from that in healthy controls. Compared to the A/A genotype, the combination of A/G and G/G genotypes did not show any significant influence on the risk of ESCC and GCA in each stratification group (Table 2).
| A/A | A/G+G/G | aOR (95%CI)3 | |
| Overall | |||
| Normal | 267 (60.3) | 176 (39.7) | |
| ESCC | 117 (57.6) | 86 (42.4) | 0.92 (0.76–1.11) |
| GCA | 93 (61.2) | 59 (38.8) | 0.95 (0.61–1.46) |
| Nonsmoker1 | |||
| Normal | 181 (62.4) | 109 (37.6) | |
| ESCC | 52 (61.2) | 33 (38.8) | 0.89 (0.67–1.18) |
| GCA | 26 (59.1) | 18 (40.9) | 0.79 (0.39–1.63) |
| Smoker | |||
| Normal | 80 (55.6) | 64 (44.4) | |
| ESCC | 61 (57.5) | 45 (42.5) | 1.01 (0.77–1.33) |
| GCA | 54 (61.4) | 34 (38.6) | 1.11 (0.61–2.02) |
| Negative family history2 | |||
| Normal | 168 (60.2) | 111 (39.8) | |
| ESCC | 48 (56.5) | 37 (43.5) | 0.92 (0.70-1.20) |
| GCA | 30 (63.8) | 17 (36.2) | 1.10 (0.54-2.22) |
| Positive family history | |||
| Normal | 99 (60.4) | 65 (39.6) | |
| ESCC | 56 (60.2) | 37 (39.8) | 0.96 (0.71–1.29) |
| GCA | 40 (54.0) | 34 (46.0) | 0.71 (0.37–1.36) |
Cixian County and Shexian County lie in Taihang Mountain located at the southern part of Hebei Province. These two counties are famous in the world for their high-incidence and mortality of esophageal cancer. GCA, which was formerly registered as esophageal cancer or gastric cancer, has been diagnosed independently in very recent years, due to the improvement in early endoscopic screening and pathologic diagnosis. It has been suggested by our epidemiological data that GCA is also a prevalent tumor type in Cixian County and Shexian County; its incidence is about one-third of ESCC in these counties. Therefore, we selected ESCC and GCA patients as well as demographically matched healthy controls as the resources of this population-based case control study.
In the present study, smoking, which was considered as one of the important risk factors in Western countries, was also suggested to significantly increase the risk of ESCC and GCA development. In addition, the study strongly supported that the genetic background might play an additional role in the development of these two tumor types. Therefore, smoking control may play an important role in the prevention of ESCC and GCA in this region. In addition, individuals with a family history of UGIC should pay more attention to their living habits and undergo a regular endoscopic inspection to prevent and early detect ESCC or GCA.
In recent years, host-dependent susceptibility to ESCC and GCA has been widely exploited especially in China[8]. Some polymorphic genes encoding metabolic enzymes, cell cycle regulators and mismatch repair enzymes, such as aldehyde dehydrogenase-2 (ALDH2)[17], cytochrome P450 (CYP)[18], glutathione S-transferase (GST), methylenetetrahydrofolate reductase (MTHFR)[8], NAD (P) H: quinone oxidoreductase 1 (NQO1)[19] have been found to be able to modify the susceptibility to chemically induced cancers including esophageal and gastric cardiac cancer. Therefore, these polymorphic genes may be used as predicative parameters for screening individuals at a high risk of ESCC and GCA.
Polymorphisms in cytokine genes might be associated with functional differences in cytokine transcription and could alter clinical performance in a variety of diseases[10,14]. IL-10 is a cytokine with anti-inflammatory and B-cell-stimulating activity. It may also influence tumor development via its action on pathways of tumor angiogenesis. It has been reported that the IL-10-1082 A/A genotype (low producer of IL-10) significantly increased in prostate cancer[14] and cutaneous malignant melanoma[20]. In contrast, high levels of IL-10 were tumor promoting and elevated serum IL-10 levels were observed in patients with various solid tumors[21]. However, in this population-based case-control study, difference in IL-10-G1082A genotype distribution was not found between ESCC or GCA patients and healthy controls, as well as in the stratification analyses according to smoking status (never smoking or current and ever smoking) and family history of UGIC, suggesting that although IL-10-1082 polymorphism has been correlated with some cancer types, this genetic alteration may not be associated with the susceptibility to ESCC and GCA.
In summary, the findings in this study indicate that IL-10-1082 promoter polymorphism might not be used as a stratification marker to predicate the susceptibility to ESCC and GCA in North China.
We thank the patients and control individuals for taking part in this study. We also thank Mr. Li-Wei Zhang, Mr. Xiao-Qing Guo and Mr. Ming-Li Wu in the Fourth Affiliated Hospital of Hebei Medical University and Mr. Bao-Shan Zhao, Mr. Zhong-Shu Liu in Cancer Prevention and Control Institute of Shexian County, and Mr. Fan-Shu Meng in Cancer Prevention and Control Institute of Cixian County, China, for their assistance in recruiting study subjects.
Edited by Wang XL
| 2. | Yokokawa Y, Ohta S, Hou J, Zhang XL, Li SS, Ping YM, Nakajima T. Ecological study on the risks of esophageal cancer in Ci-Xian, China: the importance of nutritional status and the use of well water. Int J Cancer. 1999;83:620-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 3. | Launoy G, Milan CH, Faivre J, Pienkowski P, Milan CI, Gignoux M. Alcohol, tobacco and oesophageal cancer: effects of the duration of consumption, mean intake and current and former consumption. Br J Cancer. 1997;75:1389-1396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Cai L, Yu SZ, Zhang ZF. Helicobacter pylori infection and risk of gastric cancer in Changle County,Fujian Province,China. World J Gastroenterol. 2000;6:374-376. [PubMed] |
| 5. | Lavergne D, de Villiers EM. Papillomavirus in esophageal papillomas and carcinomas. Int J Cancer. 1999;80:681-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 6. | Chang-Claude J, Becher H, Blettner M, Qiu S, Yang G, Wahrendorf J. Familial aggregation of oesophageal cancer in a high incidence area in China. Int J Epidemiol. 1997;26:1159-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Zhang W, Bailey-Wilson JE, Li W, Wang X, Zhang C, Mao X, Liu Z, Zhou C, Wu M. Segregation analysis of esophageal cancer in a moderately high-incidence area of northern China. Am J Hum Genet. 2000;67:110-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Song C, Xing D, Tan W, Wei Q, Lin D. Methylenetetrahydrofolate reductase polymorphisms increase risk of esophageal squamous cell carcinoma in a Chinese population. Cancer Res. 2001;61:3272-3275. [PubMed] |
| 9. | Bidwell J, Keen L, Gallagher G, Kimberly R, Huizinga T, McDermott MF, Oksenberg J, McNicholl J, Pociot F, Hardt C. Cytokine gene polymorphism in human disease: on-line databases, supplement 1. Genes Immun. 2001;2:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 197] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 10. | McCarron SL, Edwards S, Evans PR, Gibbs R, Dearnaley DP, Dowe A, Southgate C, Easton DF, Eeles RA, Howell WM. Influence of cytokine gene polymorphisms on the development of prostate cancer. Cancer Res. 2002;62:3369-3372. [PubMed] |
| 11. | Kikuchi S. Epidemiology of Helicobacter pylori and gastric cancer. Gastric Cancer. 2002;5:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Siekiera U, Jarosz-Chobot P, Janusz J, Koehler B. Polymorphism of TNF-alpha (308 A/G), IL-10 (1082 A/G, 819 C/T 592 A/C), IL-6 (174 G/C), and IFN-gamma (874 A/T); genetically conditioned cytokine synthesis level in children with diabetes type 1. Endokrynol Diabetol Chor Przemiany Materii Wieku Rozw. 2002;8:29-34. [PubMed] |
| 13. | Nagata J, Kijima H, Hatanaka H, Tokunaga T, Takagi A, Mine T, Yamazaki H, Nakamura M, Ueyama Y. Correlation between interleukin 10 and vascular endothelial growth factor expression in human esophageal cancer. Int J Mol Med. 2002;10:169-172. [PubMed] |
| 14. | Roh JW, Kim MH, Seo SS, Kim SH, Kim JW, Park NH, Song YS, Park SY, Kang SB, Lee HP. Interleukin-10 promoter polymorphisms and cervical cancer risk in Korean women. Cancer Lett. 2002;184:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85:1457-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 913] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 16. | Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13387] [Cited by in RCA: 14448] [Article Influence: 390.5] [Reference Citation Analysis (0)] |
| 17. | Matsuo K, Hamajima N, Shinoda M, Hatooka S, Inoue M, Takezaki T, Tajima K. Gene-environment interaction between an aldehyde dehydrogenase-2 (ALDH2) polymorphism and alcohol consumption for the risk of esophageal cancer. Carcinogenesis. 2001;22:913-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 146] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Tan W, Song N, Wang GQ, Liu Q, Tang HJ, Kadlubar FF, Lin DX. Impact of genetic polymorphisms in cytochrome P450 2E1 and glutathione S-transferases M1, T1, and P1 on susceptibility to esophageal cancer among high-risk individuals in China. Cancer Epidemiol Biomarkers Prev. 2000;9:551-556. [PubMed] |
| 19. | Zhang J, Schulz WA, Li Y, Wang R, Zotz R, Wen D, Siegel D, Ross D, Gabbert HE, Sarbia M. Association of NAD(P)H: quinone oxidoreductase 1 (NQO1) C609T polymorphism with esophageal squamous cell carcinoma in a German Caucasian and a northern Chinese population. Carcinogenesis. 2003;24:905-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Howell WM, Turner SJ, Bateman AC, Theaker JM. IL-10 promoter polymorphisms influence tumour development in cutaneous malignant melanoma. Genes Immun. 2001;2:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Fortis C, Foppoli M, Gianotti L, Galli L, Citterio G, Consogno G, Gentilini O, Braga M. Increased interleukin-10 serum levels in patients with solid tumours. Cancer Lett. 1996;104:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 3.9] [Reference Citation Analysis (0)] |