Published online Feb 14, 2005. doi: 10.3748/wjg.v11.i6.854
Revised: April 9, 2004
Accepted: May 29, 2004
Published online: February 14, 2005
AIM: To investigate the relationship between the polymorphism of class II transactivator (CIITA) gene promoters and chronic hepatitis B (CHB).
METHODS: Genomic DNA was prepared from peripheral blood leukocytes. Promoters I, III and IV of gene were analyzed respectively with polymerase chain reaction single strand conformation polymorphism (PCR-SSCP) in 65 patients with CHB, 26 patients with acute hepatitis B (AHB) and 85 normal controls.
RESULTS: No abnormal migration was found in PCR-SSCP analysis of the three promoters in the three groups. Also, no sequential difference was observed at the three promoters among the CHB patients, AHB patients and normal controls.
CONCLUSION: No polymorphism in promoters I, III and IV of CIITA gene exists in CHB patients, ABH patients and normal controls, suggesting that the promoter of CIITA gene might be a conserved domain.
- Citation: Zhao YR, Gong L, He YL, Liu F, Lu C. Relationship between polymorphism of class II transactivator gene promoters and chronic hepatitis B. World J Gastroenterol 2005; 11(6): 854-857
- URL: https://www.wjgnet.com/1007-9327/full/v11/i6/854.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i6.854
Major histocompatibility complex (MHC) class II molecules play a central role in immune responses, and the regulation of this family of genes occurs primarily at the transcriptional level. The class II transactivator (CIITA), a non-DNA-binding protein, is the master regulator of the MHC class II gene expression[1-5]. Human CIITA is encoded on chromosome 16, and the initial CIITA cDNA encodes an 1130-aminoacid protein. CIITA is the major rate-limiting factor for both constitutive and interferon-γ-induced expression of MHC class II gene. Furthermore, the expression of MHC class II is quantitatively controlled at the CIITA level[4,6]. Human CIITA gene expression is controlled by 4 distinct promoters (PI to PIV), and they are cell-type-specific and have different activation profiles, leading to multiple CIITA transcripts with different first exons, and 3 of the forms predominating. Promoter I and promoter III direct dendritic cell and B-cell-specific expressions respectively, whereas promoter IV mediates IFN-γ-inducible expression[7-10]. Recently, it has been shown that CIITA plays an important role in infectious diseases, cancer and autoimmune diseases[11-16]. Infection with hepatitis B virus (HBV) may result in different clinical outcomes[17]. There is strong evidence in HBV infection that host genetic factors play a major role in determining the outcome of the infection[18-23]. Therefore, to explore the relationship between polymorphism of CIITA gene promoters and chronic hepatitis B(CHB), we analyzed the sequence variability at promoters of CIITA transcription factor in CHB patients and compared with acute hepatitis B (AHB) patients and normal subjects by SSCP analysis of the PCR-amplified CIITA promoter region.
Sixty-five patients (42 men and 23 women, mean age, 35.5 years) with CHB and 26 patients (18 men and 8 women, mean age, 32.3 years) with AHB, and 95 healthy blood donors (62 men and 33 women, mean age, 33.4 years) from the First Hospital of Xi’an Jiaotong University, Xi’an, China, were included in this study. The diagnosis of all the cases was made according to the criteria established on the Viral Hepatitis National Conference held in 2000 in Xi’an, China[24]. All the patients and controls were Chinese Han nationals without relatives from Xi’an.
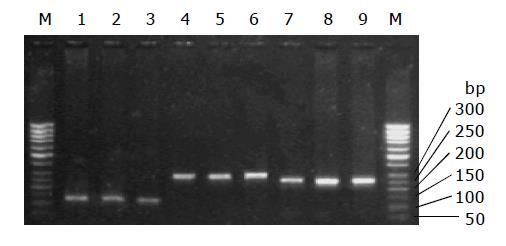
Genomic DNA was isolated from whole blood by the salt precipitation method, and dissolved in TE buffer (10 mmol/L Tris-HCl, 1 mmol/L EDTA, pH 8.0) for polymerase chain reaction (PCR) analysis. The designed primers were synthesized by BioAsia Company, Shanghai, China. The specific primer sequences of promoters I, III and IV of C II TA gene are shown in Table 1. The PCR mixture (total volume 25 μL) contained 50-100 ng of genomic DNA (0.5-1 μL), 2.5 μL of 10 mmol/L PCR buffer, 2.5 μL of 25 mmol/L MgCl2, 2 μL of 2.5 mmol/L dNTPs, 1 μL each of two fragment-specific primers, 15 μL of triple-distilled water, and 1 unit of Taq DNA polymerase (Promega, USA). The PCR mixtures were pre-denatured at 94 °C for 1 min, followed by 30 cycles of denaturation at 94 °C for 40 s, annealing at 60 °C for 40 s, and extension at 72 °C for 40 s, and the final extension at 72 °C for 5 min. The amplified fragments were run on a 15 g/L agarose gel, and confirmed to be existent (Figure 1).
| Promoters | Primer sequence | Position | Region amplified | Annealing temperature (°C) |
| 1 Sense | 5’-TGGAGTCTGAATCAACCCAA-3’ | 1.1881188 | 120 bp | 58 |
| 1 Antisense | 5’-AGGGAACCTCTGCAATTTAT-3’ | 20 | ||
| 3 Sense | 5’-TGCAGAAGGTGGCAGATATT -3’ | 1.0629139 | 298 bp | 56 |
| 3 Antisense | 5’-CAAGCTAAGCCAACATGCAA-3’ | 1.7916667 | ||
| 4 Sense | 5’-GGTTGGACTGAGTTGGAGAGA-3’ | 1.0598802 | 257 bp | 60 |
| 4 Antisense | 5’- GGAGCAACCAAGCACCTACT-3’ | 1.1938776 |
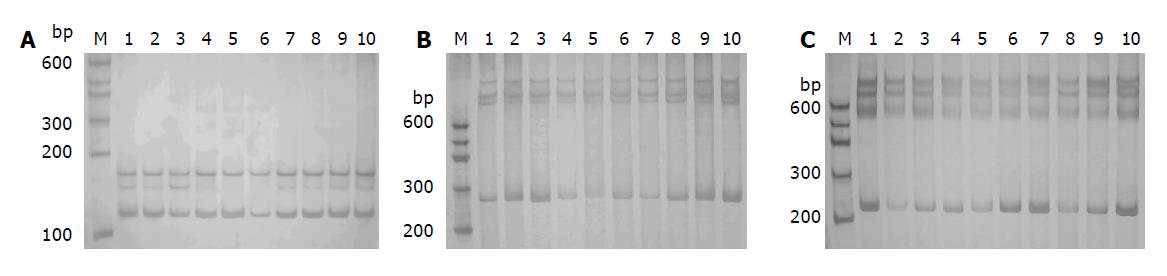
Two microliters of each PCR product was mixed with 8 μL of denaturing dye (950 mL/L formamide, 10 mmol/L NaOH, 0.5 g/L bromophenol blue, 0.5 g/L xylene cyanol), denatured at 96 °C for 5 min, and transferred into an ice-cold water bath for 3 min. The PCR products were resolved on an 80 g/L polyacrylamide gel under non-denaturing conditions in 1 g/L TBE buffer. Electrophoresis was performed at 250 V and at 4 °C for 2-3 h. Silver staining was done by fixing the gel in 100 g/L glacial acetic acid for 20 min, rinsed 3 times with ultra-pure water for 2 min each, immersed in 1 g/L AgNO3 solution for 30 min, and finally the gel was rinsed and developed with 30 g/L Na2CO3 till the bands appeared (Figure 2 A-C). All the reagents were of analytical grade.
The PCR products, which showed mobility shift in SSCP, were extracted from a 10 g/L agarose gel and column purified using a QIAquick gel extraction kit (Qiagen, Hilden, Germany), and direct sequencing was performed by BioAsia Company, Shanghai, China.
The increment of all DNA samples from the CHB patients, AHB patients and normal controls was a single strand with 120, 298 and 257 bp in length respectively, which indicated that a large fragment insertion and deletion did not exist in the region of CIITA gene promoters I, III and IV among the subjects.
For each promoter, identical SSCP patterns were detected in PCR products derived from all the subjects, whether CHB patients, AHB patients or healthy controls, with no evidence of polymorphism. Selective DNA bands detected by SSCP analysis randomly and direct DNA sequencing conformed to this result.
Thus, in the samples analyzed in the present study, no difference was found at CIITA gene promoters I, III and IV among CHB patients, AHB patients and healthy controls. The three CIITA promoters resulted in no polymorphism.
It has been estimated that HBV is present in a reservoir of more than 130 million chronic carriers, accounting for more than 10% of Chinese population[25]. Infection with HBV can cause a broad spectrum of diseases, including asymptomatic HBV carriers, acute hepatitis, chronic hepatitis, liver cirrhosis and primary hepatocellular carcinoma. Virological factors may play a role in the outcome of the infection, while infection with the same HBV virus has been found to cause various clinical outcomes in different patients suggesting that there is a strong immunogenic component to affect the individual susceptibility to HBV. So far, histocompatibility leukocyte antigen (HLA)[26-29], tumor necrosis factor alpha (TNF-α)[30], interleukin-6 (IL-6)[31], interleukin-10 (IL-10)[32,33], mannose-binding lectin (MBL) gene[34], and vitamin D receptor[35] have been investigated as potential candidate genes. However, no single allele has been clearly associated with HBV persistence or disease severity. Further study is needed to clarify these preliminary associations and to identify other potential candidate genes.
CIITA is encoded by the activator of immune response 1 (AIR-1) locus[36]. It was first discovered in a rare, severe immunodeficiency-bare lymphocyte syndrome (BLS), and the expression of CIITA was lacking in a BLS complementation of group[1]. It has been shown that CIITA can activate other genes involved in antigen presentation, such as the invariant chain, HLA-DM and MHC class I[37], and can affect HIV long terminal repeat expression. Impaired induction of CIITA might decrease IFN-γ-induced HLA-DR expression in cultured thymic epithelial cells (TEC) derived from thymoma and affect CD4+ T cell development[38]. CIITA could compete with nuclear factor of activated T cells (NF-AT) to bind to the CREB binding protein (CBP/p300) and inhibit Th2-type cytokine production during Th1 cell differentiation to prevent interleukin-4 (IL-4) gene transcription[39]. Quan et al[40] investigated the underlying genetic defect in an immunodeficient patient who presented with recurrent bacterial infections in his late twenties and demonstrated that a single amino acid substitution, phenylalanine to serine, in the COOH-terminal portion of the CIITA sequence was correlated with reduced transcription of MHC class II genes. Marten et al[41] transfected dendritic cells (DCs) with the CIITA gene efficiently and found an increase in antitumoral immunostimulatory capacity of DCs. Sartoris et al[42] transfected an expressible CIITA cDNA in uninducible hepatocarcinoma cell lines, and found that there was a very high expression of HLA class II in the transfected cell surface and they had acquired antigen processing and presentation capacity.
The availability of CIITA appears generally essential for MHC class II gene expression, and hence its transcriptional regulatory mechanisms are of fundamental importance for a correct homeostasis of the immune response. Therefore, it is possible to hypothesize that variability at the CIITA-encoding locus, AIR-1, could constitute an additional source of susceptible traits to autoimmune diseases. Sartoris et al analyzed the sequence variation at AIR-1/CIITA promoters by PCR-SSCP in 88 Caucasians from the Northeast of Italy, including 23 insulin-dependent diabetes mellitus (IDDM), 30 rheumatoid arthritis (RA) patients, compared with a sample of 19 unaffected normal controls and 16 unaffected IDDM family members, and they did not observe any sequence difference at the four AIR-1/CIITA promoters between autoimmune patients and normal controls. Rasmussen et al searched for genetic susceptibility to multiple sclerosis (MS). Polymorphism screening based upon detection of single strand conformational changes (SSCP analysis) followed by sequencing revealed six single nucleotide variations, i.e., one in the promoter-utilized by B cells and five in the 3 untranslated region (UTR) of the gene. The distribution of CIITA alleles did not differ between MS patients and control subjects. After sub-grouping the patients into relapsing-remitting MS and primary progressive MS, the distribution of promoter alleles at the BslI polymorphic site in the latter of the two patient groups differed from that of healthy control subjects. It is suggested that there is a weak association between one of the alleles at this site and primary progressive MS.
We studied the sequence variability at promoter I, III and IV of CIITA transcription factor in CHB patients, ABH patients and normal controls by SSCP analysis of the three PCR-amplified CIITA promoter regions to assess whether there was a relationship between polymorphism of CIITA gene promoters and the chronicity of hepatitis B. The similar report on the relationship between polymorphism of CIITA gene promoters and CHB has not been found abroad and in our country. We found no polymorphic variation at the three CIITA promoters significantly associated with CHB. Due to the importance of CIITA in the class II gene expression, the polymorphism in the coding region and 3’UTR of this gene needs to be studied further. We suppose that the promoter region of CIITA gene may be a conserved domain and the variability at CIITA promoters might not improve the overall potentiality of the system against pathogens. Furthermore, studies on a large number of cases will reveal significance of polymorphism in CIITA promoters and its relation with CHB.
Edited by Wang XL, Ma JY and Kumar M
| 1. | Steimle V, Otten LA, Zufferey M, Mach B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome). Cell. 1993;75:135-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 145] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Ting JP, Trowsdale J. Genetic control of MHC class II expression. Cell. 2002;109 Suppl:S21-S33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 433] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 3. | van den Elsen PJ, Holling TM, Kuipers HF, van der Stoep N. Transcriptional regulation of antigen presentation. Curr Opin Immunol. 2004;16:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Schnappauf F, Hake SB, Camacho Carvajal MM, Bontron S, Lisowska-Grospierre B, Steimle V. N-terminal destruction signals lead to rapid degradation of the major histocompatibility complex class II transactivator CIITA. Eur J Immunol. 2003;33:2337-2347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Nagarajan UM, Bushey A, Boss JM. Modulation of gene expression by the MHC class II transactivator. J Immunol. 2002;169:5078-5088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Otten LA, Steimle V, Bontron S, Mach B. Quantitative control of MHC class II expression by the transactivator CIITA. Eur J Immunol. 1998;28:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Muhlethaler-Mottet A, Otten LA, Steimle V, Mach B. Expression of MHC class II molecules in different cellular and functional compartments is controlled by differential usage of multiple promoters of the transactivator CIITA. EMBO J. 1997;16:2851-2860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 412] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 8. | Lennon AM, Ottone C, Rigaud G, Deaven LL, Longmire J, Fellous M, Bono R, Alcaïde-Loridan C. Isolation of a B-cell-specific promoter for the human class II transactivator. Immunogenetics. 1997;45:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Wong AW, Ghosh N, McKinnon KP, Reed W, Piskurich JF, Wright KL, Ting JP. Regulation and specificity of MHC2TA promoter usage in human primary T lymphocytes and cell line. J Immunol. 2002;169:3112-3119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Pai RK, Askew D, Boom WH, Harding CV. Regulation of class II MHC expression in APCs: roles of types I, III, and IV class II transactivator. J Immunol. 2002;169:1326-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Abendroth A, Slobedman B, Lee E, Mellins E, Wallace M, Arvin AM. Modulation of major histocompatibility class II protein expression by varicella-zoster virus. J Virol. 2000;74:1900-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Kanazawa S, Okamoto T, Peterlin BM. Tat competes with CIITA for the binding to P-TEFb and blocks the expression of MHC class II genes in HIV infection. Immunity. 2000;12:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 217] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Accolla RS, Mazza S, De Lerma Barbaro A, De Maria A, Tosi G. The HLA class II transcriptional activator blocks the function of HIV-1 Tat and inhibits viral replication. Eur J Immunol. 2002;32:2783-2791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Yazawa T, Ito T, Kamma H, Suzuki T, Okudela K, Hayashi H, Horiguchi H, Ogata T, Mitsui H, Ikeda M. Complicated mechanisms of class II transactivator transcription deficiency in small cell lung cancer and neuroblastoma. Am J Pathol. 2002;161:291-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Sartoris S, Brendolan A, Degola A, Testi MG, Chignola R, Scarpa A, Scardoni M, Contreas G, Pinelli L, Lunardi C. Analysis of CIITA encoding AIR-1 gene promoters in insulin-dependent diabetes mellitus and rheumatoid arthritis patients from the northeast of Italy: absence of sequence variability. Hum Immunol. 2000;61:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Rasmussen HB, Kelly MA, Clausen J. Genetic susceptibility to multiple sclerosis: detection of polymorphic nucleotides and an intron in the 3' untranslated region of the major histocompatibility complex class II transactivator gene. Hum Immunol. 2001;62:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Iino S. Natural history of hepatitis B and C virus infections. Oncology. 2002;62 Suppl 1:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Wang FS. Current status and prospects of studies on human genetic alleles associated with hepatitis B virus infection. World J Gastroenterol. 2003;9:641-644. [PubMed] |
| 19. | McNicholl JM, Cuenco KT. Host genes and infectious diseases. HIV, other pathogens, and a public health perspective. Am J Prev Med. 1999;16:141-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Dean M, Carrington M, O'Brien SJ. Balanced polymorphism selected by genetic versus infectious human disease. Annu Rev Genomics Hum Genet. 2002;3:263-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Hill AV. The immunogenetics of human infectious diseases. Annu Rev Immunol. 1998;16:593-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 353] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 22. | Kwiatkowski D. Genetic dissection of the molecular pathogenesis of severe infection. Intensive Care Med. 2000;26 Suppl 1:S89-S97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Thursz M. Genetic susceptibility in chronic viral hepatitis. Antiviral Res. 2001;52:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Branch society of infectious disease and parasitology and branch society of hepatology of Chinese medical association. National preventiont and treatment profiles of viral hepatitis (2000). Zhonghua Chuanranbing Zazhi. 2001;19:56-62. |
| 25. | Zhang S, Chen Y. HBV serum marker detection and relative factor analysis of 2925 new students. Public Health. 1998;112:257-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Cheng YQ, Lin JS, Huang LH, Tian DY, Xiong P. The association of HLA-DRB1 allele polymorphism with the genetic susceptibility to liver cirrhosis due to hepatitis B virus. Zhonghua Yixue Yixhuanxue Zazhi. 2003;20:247-249. |
| 27. | Höhler T, Gerken G, Notghi A, Lubjuhn R, Taheri H, Protzer U, Löhr HF, Schneider PM, Meyer zum Büschenfelde KH, Rittner C. HLA-DRB1*1301 and *1302 protect against chronic hepatitis B. J Hepatol. 1997;26:503-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 128] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Thio CL, Carrington M, Marti D, O'Brien SJ, Vlahov D, Nelson KE, Astemborski J, Thomas DL. Class II HLA alleles and hepatitis B virus persistence in African Americans. J Infect Dis. 1999;179:1004-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Karan MA, Tascioglu NE, Ozturk AO, Palanduz S, Carin M. The role of HLA antigens in chronic hepatitis B virus infection. J Pak Med Assoc. 2002;52:253-256. [PubMed] |
| 30. | Kim YJ, Lee HS, Yoon JH, Kim CY, Park MH, Kim LH, Park BL, Shin HD. Association of TNF-alpha promoter polymorphisms with the clearance of hepatitis B virus infection. Hum Mol Genet. 2003;12:2541-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Park BL, Lee HS, Kim YJ, Kim JY, Jung JH, Kim LH, Shin HD. Association between interleukin 6 promoter variants and chronic hepatitis B progression. Exp Mol Med. 2003;35:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Ben-Ari Z, Mor E, Papo O, Kfir B, Sulkes J, Tambur AR, Tur-Kaspa R, Klein T. Cytokine gene polymorphisms in patients infected with hepatitis B virus. Am J Gastroenterol. 2003;98:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 180] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 33. | Miyazoe S, Hamasaki K, Nakata K, Kajiya Y, Kitajima K, Nakao K, Daikoku M, Yatsuhashi H, Koga M, Yano M. Influence of interleukin-10 gene promoter polymorphisms on disease progression in patients chronically infected with hepatitis B virus. Am J Gastroenterol. 2002;97:2086-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Song le H, Binh VQ, Duy DN, Jüliger S, Bock TC, Luty AJ, Kremsner PG, Kun JF. Mannose-binding lectin gene polymorphisms and hepatitis B virus infection in Vietnamese patients. Mutat Res. 2003;522:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Bellamy R, Ruwende C, Corrah T, McAdam KP, Thursz M, Whittle HC, Hill AV. Tuberculosis and chronic hepatitis B virus infection in Africans and variation in the vitamin D receptor gene. J Infect Dis. 1999;179:721-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 273] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 36. | Accolla RS, Jotterand-Bellomo M, Scarpellino L, Maffei A, Carra G, Guardiola J. aIr-1, a newly found locus on mouse chromosome 16 encoding a trans-acting activator factor for MHC class II gene expression. J Exp Med. 1986;164:369-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 87] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Martin BK, Chin KC, Olsen JC, Skinner CA, Dey A, Ozato K, Ting JP. Induction of MHC class I expression by the MHC class II transactivator CIITA. Immunity. 1997;6:591-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 167] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Kadota Y, Okumura M, Miyoshi S, Kitagawa-Sakakida S, Inoue M, Shiono H, Maeda Y, Kinoshita T, Shirakura R, Matsuda H. Altered T cell development in human thymoma is related to impairment of MHC class II transactivator expression induced by interferon-gamma (IFN-gamma). Clin Exp Immunol. 2000;121:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Sisk TJ, Gourley T, Roys S, Chang CH. MHC class II transactivator inhibits IL-4 gene transcription by competing with NF-AT to bind the coactivator CREB binding protein (CBP)/p300. J Immunol. 2000;165:2511-2517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Quan V, Towey M, Sacks S, Kelly AP. Absence of MHC class II gene expression in a patient with a single amino acid substitution in the class II transactivator protein CIITA. Immunogenetics. 1999;49:957-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Märten A, Ziske C, Schöttker B, Weineck S, Renoth S, Buttgereit P, Schakowski F, König S, von Rücker A, Allera A. Transfection of dendritic cells (DCs) with the CIITA gene: increase in immunostimulatory activity of DCs. Cancer Gene Ther. 2001;8:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Sartoris S, Valle MT, Barbaro AL, Tosi G, Cestari T, D'Agostino A, Megiovanni AM, Manca F, Accolla RS. HLA class II expression in uninducible hepatocarcinoma cells after transfection of AIR-1 gene product CIITA: acquisition of antigen processing and presentation capacity. J Immunol. 1998;161:814-820. [PubMed] |