Published online Feb 14, 2005. doi: 10.3748/wjg.v11.i6.810
Revised: July 11, 2004
Accepted: September 19, 2004
Published online: February 14, 2005
AIM: To assess the adhesion- and abscess-reducing capacities of various concentrations of polysaccharides derived from fungus, Phellinus gilvus (PG) or Phellinus linteus (PL) in a rat peritonitis model.
METHODS: In 96 SD rats, experimental peritonitis was induced using the cecal ligation and puncture model (CLP). Rats were randomly assigned to 8 groups; Ringer’s lactate solution (RL group), hyaluronic acid (HA group), 0.025%, 0.25%, and 0.5% polysaccharides from PG (PG0.025, 0.25, and 0.5 groups), and PL (PL0.025, 0.25, and 0.5 groups). Adhesions and abscesses were noted at 7 d after CLP. RT-PCR assay was performed to assess the cecal tissue.
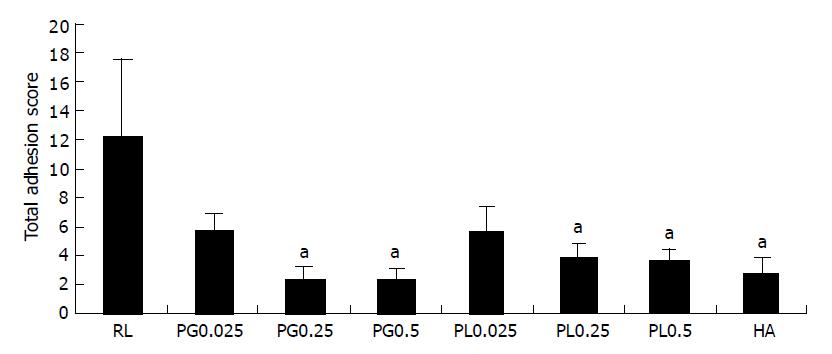
RESULTS: Adhesion formation was significantly reduced in PG0.25, 0.5, PL0.25, 0.5, and HA groups (2.5±0.7, 2.4±0.7, 3.8±1.0, 3.6±0.8, and 2.7±1.1, P<0.05). The incidence of abscesses was significantly reduced in all treated groups compared to RL group (58%, P<0.05). The urokinase-type plasminogen activator (uPA) gene expression was greatly up-regulated by increasing the concentration of polysaccharides. The urokinase-type plasminogen activator receptor (uPAR) and tumor necrosis factor (TNF)-α mRNA were highly expressed in PG0.25, 0.5, PL0.25, and 0.5 groups.
CONCLUSION: We concluded that 0.5% polysaccharide derived from PG and PL was the optimal concentration in preventing adhesion and abscess formation and may act by modulating activity of uPA and TNF-α in a rat peritonitis model.
-
Citation: Bae JS, Jang KH, Jin HK. Comparison of intraperitoneal anti-adhesive polysaccharides derived from
Phellinus mushrooms in a rat peritonitis model. World J Gastroenterol 2005; 11(6): 810-816 - URL: https://www.wjgnet.com/1007-9327/full/v11/i6/810.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i6.810
Intraperitoneal adhesions and abscesses are a major cause of morbidity and mortality in the adult intensive care unit. They are caused mainly by previous surgery and abdominal inflammation[1,2]. Abdominal infection is accompanied by peritoneal inflammation, including exudation of fibrinogen and fibrin formation into the abdominal cavity. In infectious conditions, these fibrin deposits may become a nidus for abscesses[3] and in turn become fibrous adhesions. Therefore peritoneal fibrinolytic system is crucial in preventing adhesion and abscess formation. Activation of fibrinolytic system results in the conversion of plasminogen into plasmin. The conversion is activated directly by tissue-type plasminogen activators (tPA) and uPA[4,5]. Various cells produce tPA, including endothelial cells, mesothelial cells, and macrophages. The uPA is also produced by the same cells and is equally effective in the degradation of fibrin[6].
Numerous agents have been investigated in the prevention of adhesion and abscess formation. Recently, it was reported that beta-glucan in polysaccharides from chemical was capable of reducing the frequency of adhesion by preventing with beta-glucanase[7]. It is a potent macrophage stimulator that enhances macrophage cytotoxicity and phagocytic capacity and induces production of TNF-α[8,9].
We have also previously demonstrated that 0.025% polysaccharides derived from PG and PL reduced both intraperitoneal adhesion and abscess formation by modulating activity of uPA and TNF-α produced from activated macrophages in a rat peritonitis model[10]. However, comparison of adhesion- and abscess-reducing capacity by varying concentrations of polysaccharides solutions derived from PG and PL has not been demonstrated to date.
Therefore, in this study, we hypothesize that intraperitoneal abscesses and adhesions could be reduced by increasing concentration of polysaccharides solutions derived from PG and PL, and differences of the effect could be related to uPA and TNF-α activity produced from activated macrophages. To test this hypothesis, we investigated differences by varying concentration of polysaccharides derived from PG and PL in preventing adhesion and abscess formation in order to investigate influence of the uPA and TNF-α gene expression in a rat peritonitis model.
The fruiting body of PG was kindly provided by Gyeongbuk Agricultural Technology Administration (Daegu, Korea). A seed culture was grown in a 250-mL flask containing 50 mL of PMP medium (2.4% potato/dextrose broth plus 1% malt extract, 0.1% peptone) at 28 °C on a rotary incubator at 150 r/min for 4 d. To obtain fruiting bodies of PG, a culture was grown in oak sawdust block for 90 d. The yield of fruiting bodies was 97 g dried weight per block. PL used in this study was developed for 3 years in routine artificial mulberry cultures and purchased from Sanwhang Mushroom Co. (Andong, Korea). The fruiting body of PG and PL was cut into small pieces, dried at 40-50 °C for 48 h. It was homogenized, extracted by optimal water extraction conditions, distilled water (1:25) at 100 °C for 10 h, and concentrated at 80 °C in a rotary evaporator. The recovery procedure of the polysaccharides from the fruiting body of PG and PL followed an established method in our previous study[10,11]. The concentration of 0.025%, 0.25%, and 0.5% polysaccharides solutions was determined by measuring total sugar using the anthrone method[12] using glucose as the standard material. The 0.2% HA was prepared by adding distilled water at HA (HYAL®, Shinpoong Pharm. Co., Korea). It was filtered through a 0.22 μm membrane filter. All the materials were stored at 4 °C until used.
Ninety-six male Sprague-Dawley (SD) rats (Charles River Korea Inc., Korea) weighing 237 to 261 g were acclimated under the controlled conditions for 1 wk before the experiments. The animals were fed with commercial rat feed from Orient Inc., Korea. Food and water were provided ad libitum to the animals. The Guidelines for Animal Care and Use of Kyungpook National University approved the housing, care and use of animals, as well as procedures to minimize discomfort.
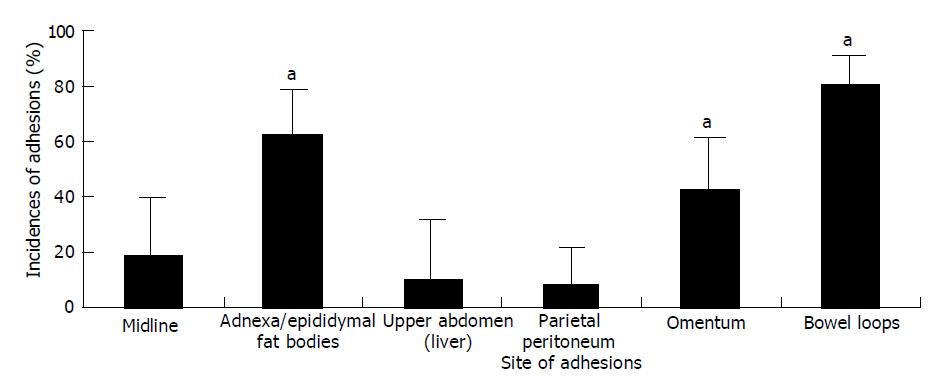
Bacterial peritonitis was induced by performing a CLP procedure according to Wichterman et al[13]. Only water was provided in the 12 h preceding the experiments. The animals were weighed and anesthetized by intramuscular injection of a combination of ketamine (100 mg/kg) and xylazine (5 mg/kg). They breathed spontaneously throughout the procedures. The abdominal skin was disinfected with 70% alcohol. All procedures were performed under sterile conditions. Routine midline celiotomy was performed with a 3-cm incision and the cecum was exposed. The cecum was ligated just distally to ileocecal valve with a 3-0 polyglactin 910 (VICRYL®, ETHICON, Inc., Johnson & Johnson Co., San Angelo, TX) suture to avoid intestinal obstruction, punctured once with a 19-G needle, squeezed gently to force out a small amount of feces, and then returned to the abdominal cavity. After closing the abdomen in two layers with 3-0 polyglactin 910 sutures, the animals received enrofloxacin (1 mg/kg) and 10 mL of isotonic sodium chloride solution subcutaneously for hydration. After 24 h, animals were weighed and the abdomen was reopened under the same anesthesia as the first celiotomy. Samples of peritoneal fluid were taken for microbiologic examination. The abdominal cavity was rinsed with 10 mL isotonic sodium chloride solution, and the cecum was resected. Before closure of the abdomen, the animals were randomly allocated to 8 groups of 12. One control group was treated with 8 mL of ringer lactate solution (RL group) and one other control group was treated with 8 mL of 0.2% HA solution (HA group) intraperitoneally through the urinary catheter. Six experimental groups were treated with 8 mL of 0.025%, 0.25%, and 0.5% polysaccharides derived from PG (PG0.025, 0.25, and 0.5 groups) and PL (PL0.025, 0.25, and 0.5 groups) intraperitoneally, respectively. All animals were given water only on the first postoperative day; standard rat chow and water ad libitum were provided on the second postoperative day. The animals were weighed again and killed with carbon dioxide asphyxiation a week after the first postoperative day. The abdomen was opened via a U-shaped incision for complete exploration. Adhesions and the incidence of abscesses were examined in a blind manner by one of us (HK Jin) according to the method of Zuhlke et al[14], whereby grade 0 means no adhesions and grade IV means firm extensive adhesions that are dissectible only with sharp instruments, with organ damage almost unavoidable. Sites of adhesions scored included the midline, adnexa/epididymal fat bodies, the upper abdomen (liver), the parietal peritoneum, the omentum, and between the bowel loops. The total score of these six locations was noted as the total adhesion score (0-24) (Table 1).
| Grade | Description |
| 0 | No adhesions |
| 1 | Filmy adhesions: gentle, blunt dissection required to free adhesions |
| 2 | Mild adhesions: aggressive blunt dissection required to free adhesions |
| 3 | Moderate adhesions: sharp dissection required to free adhesions |
| 4 | Severe adhesions: not dissectible without damaging organs |
Samples of peritoneal fluid and abscesses were taken from all animals on the second postoperative day by swabs for verification of the induced peritonitis. The swabs were immediately introduced into medium and cultured semiquantitatively in aerobic and anaerobic conditions. Samples were incubated on blood and EMB agar for aerobic culture and layered on anaerobic blood agar and incubated in a Gas-Pak jar for anaerobic culture. After 24 and 48 h of incubation at 37 °C, growing colonies were identified with standard bacteriologic techniques.
The adhesion-carrying cecal site was resected carefully. The cecal tissue was cut longitudinally to remove food contents, washed with sterile phosphate-buffered saline (PBS). Half the animals in each group were fixed in 10% formalin in PBS for histopathologic evaluation and the remaining animals in each group were stored at 80 °C for RT-PCR analysis until further processing.
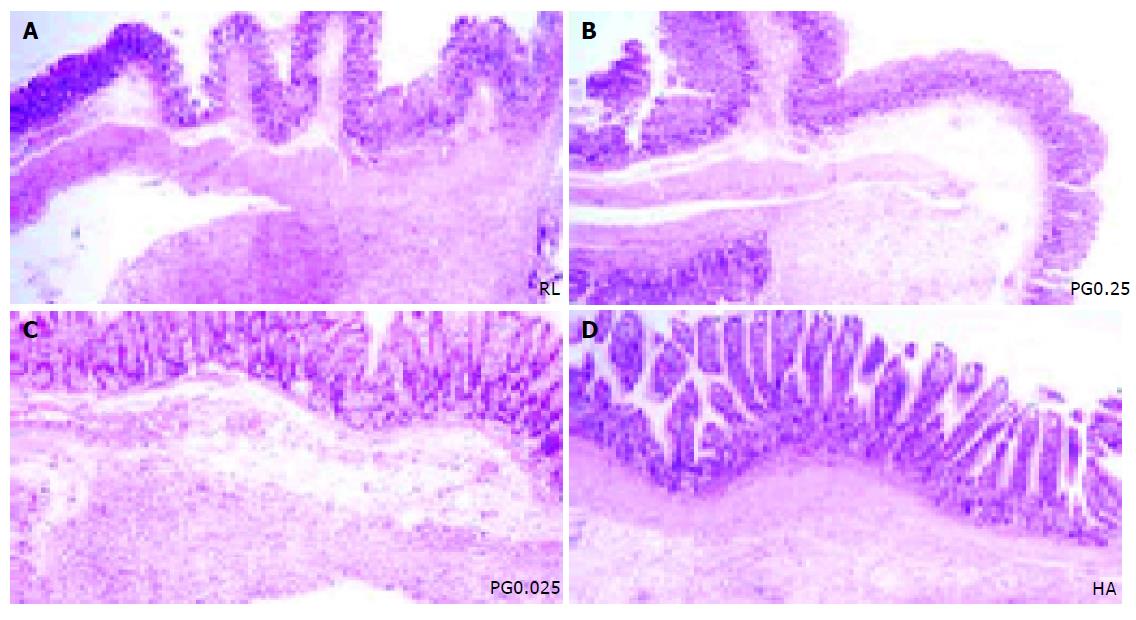
After routine tissue processing, serial sections (5 μm) were stained with hematoxylin and eosin (HE). The inflammatory reaction was assessed for each group by light microscopy. The grade of inflammation was assessed using a semi-quantitative scoring system, the inflammation grading scale[15]. Grade 1 on this scale represents a mild inflammatory reaction with giant cells, occasionally scattered lymphocytes, and plasma cells. Grade 2 represents a moderate reaction with giant cells and increased admixed lymphocytes, plasma cells, eosinophils, and neutrophils. Grade 3 represents a severe inflammatory reaction with microabscesses present.
Total cellular RNA was extracted from rat cecum using a monophasic solution of phenol and guanidine isothiocyanate (TRIzol Reagent, Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The purity and integrity of the RNA samples were assessed by A260/280 spectrophotometric measurements.
A 1 μg portion of total RNA was subjected to first-strand cDNA synthesis in a 20 μL reaction mixture containing Moloney murine leukemia virus reverse transcriptase (10 U), dNTP mixture (2.5 mmol/L concentrations of each dNTP), oligo (dT)12-18 primers (10 μmol/L), and reaction buffer as supplied with the enzyme (50 mmol/L Tris-HCl (pH 8.3), 50 mmol/L KCl, 10 mmol/L MgCl2, 0.5 mmol/L spermidine, and 10 mmol/L dithiothreitol). The samples were incubated in a TOUCHgene DNA thermal cycler (Techne (Cambridge) Limited, U.K.) at 42 °C for 60 min followed by enzyme denaturation step at 94 °C for 2 min. The reverse transcription mixture was stored at -80 °C for use in PCR. All reagents were obtained from Promega (Madison, WI).
PCR was performed on 2 μL of reverse transcriptase product using Gene Taq (Nippon Gene Co., Ltd., Toyama, Japan) containing Taq DNA polymerase, dNTPs, buffer, and 0.5 μmol/L concentrations of each gene-specific forward and reward primers (obtained from Bioneer, Daejeon, Korea) in a total volume of 50 μL. Gene-specific oligonucleotide primers were designed from published rat sequences. Primers used for amplification: TNF-α: sense, 5’-TACTGAACTTCGGGGTGATTGGTCC-3’, antisense, 5’-CAGCCTTGTCCCTTGAAGAGAACC-3’; uPA: sense, 5’-TCGTGAATCAGCCAAAGAAGGAAGAGTACG-3’, antisense, 5’-TTACAACTGACATTTTCAGGTCC-3’; uPAR: sense, 5’-CAGAACACTGTATTGAAGTGGTGACGCTCC-3’, antisense, 5’-TCCAAGCACTGATTCATTGGTCCCCG-3’; glyceraldehydes-3-phosphate dehydrogenase (GAPDH): sense, 5’-TGAAGGTCGGTGTGAACGGATTTGGC-3’, antisense, 5’-CATGTAGGCCATGAGGTCCACCAC-3’. The PCR was conducted in TOUCHgene DNA thermal cycler. After an initial denaturation at 95 °C for 5 min, amplification was conducted through 35 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C (GAPDH) or 60 °C (for all the other transcripts) for 30 s and extension at 72 °C for 45 s. Final extension was at 72 °C for 10 min followed by a final hold at 4 °C. Negative controls (PCR mixture without cDNA) and positive controls (PCR mixture with a standard cDNA sample) were included in preliminary PCR runs. Initial experiments were conducted to determine the optimal annealing temperature for each set of gene-specific primers (data not shown). The PCR products were separated by electrophoresis using 2% agarose gels stained with ethidium bromide to visualize cDNA products.
Values are expressed as mean±SD. Analysis of differences between treated groups and untreated groups was performed using analysis of variance followed by multiple comparisons and Fisher’s LSD test using the SAS statistical package (release 8.1; SAS Institute Inc., Cary, NC). Differences at P<0.05 were considered statistically significant.
Following CLP, all rats showed symptoms of peritonitis-like apathetic behavior, ocular exudates, and piloerection. These symptoms resolved within 2 d following the re-celiotomy and removal of the necrotic, perforated cecum and peritoneal lavage. Survival rates in all groups were 100% by the end of the experiment.
The mean±SD body weight of the rats was 248.1±12.3 g at the time of the first operation. The rats lost weight during peritonitis (240.2±12.8 g) and recovered weight by the end of the experiment (255.3±10.7 g) in RL, PG0.025, PL0.025, and HA groups. The differences in weight gain were not statistically significant among the groups. But, the weight gain in the PG0.25, 0.5, PL 0.25, and 0.5 groups (270.2±12.8 g) was higher than that in RL, PG0.025, PL0.025, and HA groups.
Culture results of the peritoneal fluid taken at the day of cecal resection revealed polymicrobial intraperitoneal infection. The most frequently isolated microorganisms were Escherichia coli (50.9%), Proteus species (57.5%), Staphylococcus (48.1%), Streptococcus (25%), Gram-positive Bacillus species (15%), and Klebsiella (4%). E. coli (84.7%) was the organism isolated most frequently from abdominal abscesses.
The PG polysaccharide solutions Rats treated with 0.25% and 0.5% of PG polysaccharide solutions had a significantly lower total adhesion score compared to that of the RL group (P<0.05). The total adhesion score of rats treated with the RL solution was 12.3±5.2. Group treated with 0.25% (2.5±0.7) and 0.5% (2.4±0.7) of PG polysaccharide solutions was lower than the group treated with 0.025% (5.8±1.0) solution in total adhesion score (P<0.05) (Figure 1). There was no significant difference in total adhesion score between PG0.25 and PG0.5 group. The prevention effect of adhesion of PG0.25 and 0.5 groups was slightly higher than that in PL0.25, PL0.5, and HA (2.7±1.1) groups. There was no statistical difference in total adhesion score. Eight of 12 (67%) RL-treated rats had grade IV adhesions, in contrast to only 2 of 12 (17%) PG 0.025% and 0 of 12 (0%) PG0.25 and 0.5% treated rats. The site of the adhesions did not differ among the PG groups. Most of the adhesions were found between the bowel loops (81.1%), adnexa/epididymal fat bodies (62.5%), and the omentum (43.2%) in the PG groups (P<0.05) (Figure 2).
The PL polysaccharide solution Rat treated with 0.25 and 0.5% of PL polysaccharide solutions had a significantly lower total adhesion score compared to RL group (P<0.05) (Figure 1). The prevention effect of adhesion was slightly lower than the effect of PG0.25, PG0.5, and HA groups. Group treated with 0.25% (3.8±1.0) and 0.5% (3.6±0.8) of PL polysaccharide solutions was lower than the group treated with 0.025% PL (5.6±1.8) solution in total adhesion score (P<0.05). There was no significant difference in total adhesion score between PL0.25 and PL0.5 groups like groups treated with 0.25% and 0.5% of PG polysaccharide solutions. The prevention effect of adhesion of PL0.25 and 0.5 groups was slightly lower than that in HA (2.7±1.1) groups. One of 12 (17%) PL-treated rats had grade IV adhesions. The site of the adhesions did not differ among the PL groups. Site of the adhesions was similar to the site of rats treated PG (Figure 2).
The incidence of intraperitoneal abscess significantly reduced in all treated groups (P<0.05) compared to that in RL group. No abscess occurred in rats treated with PG 0.25 and 0.5. Rats treated with HA (3 of 12, 25%) reduced the incidence of abscesses compared to RL (7 of 12, 58%).
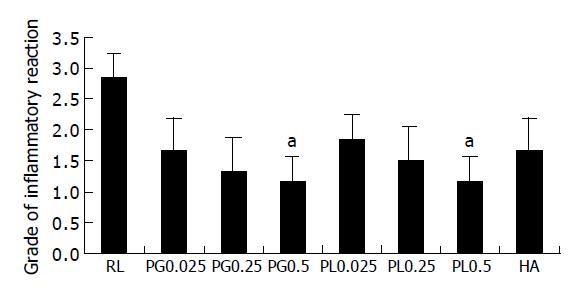
Mostly, the inflammatory reaction is dominant at mesenteric fat and serosal surface of cecum. Rats treated with PG0.25 showed markedly reduced inflammatory reaction compared to RL (Figure 3). The RL group showed increased admixed lymphocytes, plasma cells, eosinophils, and neutrophils (grade 3 on the inflammation grading scale). The grade of inflammatory response for the PG0.5 and PL0.5 groups (1.1±0.4 and 1.2±0.4) was significantly lower than the grade for the RL (2.9±0.4) (P<0.05) and slightly lower than PG0.025 and 0.25 (1.7±0.5 and 1.3±0.5), PL0.025 and 0.25 (1.8±0.4 and 1.5±0.5), and HA group (1.6±0.5) (Figure 4).
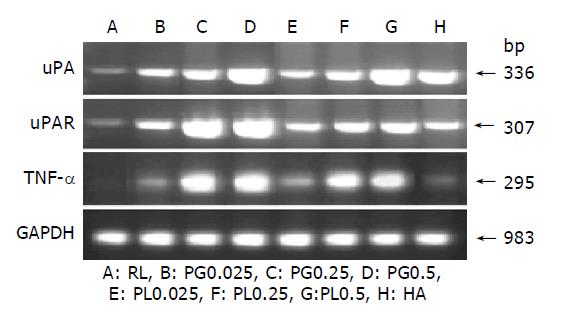
TNF-α, uPA, and uPAR mRNA expression
Experiments were carried out to demonstrate the effect of PG, PL, and HA on the gene transcription of TNF-α, uPA and uPAR. In the PG and PL treatment groups, TNF-α mRNA was highly expressed, as compared to treatment with RL in PG0.25, 0.5, PL0.25, and 0.5 groups. The level slightly increased compared to RL in PG0.025, PL0.025, and HA. The uPA gene expression was greatly up-regulated by increasing concentration of polysaccharides in the PG and PL treatment groups. In HA group, the level was highly expressed, as compared to treatment with RL. The uPA receptor (uPAR) mRNA was expressed at the highest levels by the treatment with PG0.25 and 0.5. In PL and HA groups, the level was expressed compared to RL group. The GAPDH transcript levels among all groups were the same (Figure 5).
Abdominal infections are associated with fibrin deposit, which may cause clinically significant adhesion and abscess formation. Adhesions are the most common cause of intestinal obstruction in developed countries and important cause of chronic abdominal and pelvic pain[16-19].
The CLP model has been used frequently for elucidating the pathophysiology of abdominal inflammation and developing new treatment modalities[20], because it is caused by the intraperitoneal adhesions that are promoted by surgical trauma, bacterial contamination, allergic reactions to foreign bodies left in the abdomen, and tissue ischemia. Thus, in our study, we used the CLP model that underwent cecal resection to induce adhesion and abscess formation in infectious environment.
The number of different agents has been studied in the search to prevent intraperitoneal adhesions, with variable results. Many studies showed HA decreases inflammation[21], interferes with fibrin formation[22], prevents adhesion and abscess formation[23], and stimulates fibrinolytic and TNF-α response[24,25]. In our study, we treated with 0.2% HA to prevent intraperitoneal adhesion in rats and this solution significantly reduced adhesion and abscess formation by modulating uPA and TNF-α production. This is in accordance with the results from earlier studies[23-25] for the prevention of intraperitoneal adhesions formation in rats using 0.2% HA. And, we have previously demonstrated that 0.025% polysaccharides derived from PG and PL reduced both intraperitoneal adhesion and abscess formation by modulating activity of uPA and TNF-α produced from activated macrophages in a rat peritonitis model[10]. In the present study, we treated with varying concentrations of 0.025%, 0.25%, and 0.5% of PG and PL in rats. It resulted that 0.25 and 0.5% solutions had higher adhesion- and abscess-reducing capacity than 0.025% solution in rats. The uPA gene expression was greatly up-regulated by increasing concentration of the solutions. Therefore, we think that PG and PL may act in a dose-dependent manner in the prevention of adhesion and abscess formation and the action may be determined by modulating fibrinolytic capacity of uPA produced from macrophages.
In short, PG and PL used in our study are fungi belonging to the species of Hymenochaetaceae (Basidiomycetes) and found mainly in tropical areas of America and Africa[26]. Polysaccharides isolated from them have received special attention due to their potent pharmacological activities. PL is well known as one of the most popular medicinal mushrooms due to its high anti-tumor[27] and immunostimulating activities[28]. It has been used medicinally in Korea and Japan. Recently, it was reported that polysaccharide solutions from PL and PG had anti-inflammatory activity related to arthritis, septic shock, and pulmonary inflammation[29-31]. Thus, we think that these anti-inflammatory activities of natural products like PG and PL may be beneficial in the treatment of intraperitoneal adhesion related to inflammation.
PG has other advantages over PL in that it has a very short growth period (3 mo) compared to PL (2-3 years) making it cheaper to produce and the safety of acute single orally-administered dose of PG has been demonstrated. In the present study, although it is not a statistically significant analysis, polysaccharides from PG had better capacity than PL in the prevention of adhesion and abscess formation. Therefore, this suggests an additional potential therapeutic role for PG in the treatment of inflammation in future.
In an experimental study, Bedirli et al[7] showed that beta-glucan in polysaccharides had a positive weight gain effect on the animals. In our study, although it is not a statistically significant analysis, weight gain in the 0.25% and 0.5% of PG and PL groups is higher than that in RL, HA, PG0.025, and PL0.025 groups. That is to say, it showed a positive weight gain in groups treated with high dose, 0.25% and 0.5% of polysaccharides. However, the precise mechanism of weight gain in these animals is uncertain. We postulate that this could be related to a difference in the mechanism by which adhesion formation is reduced.
Many studies showed that beta-glucan in polysaccharides is a potent stimulator of macrophage functions and it induces TNF-α production in wound tissue[8,9]. But the role of TNF-α in adhesion formation is not clear. More recently, Reijnen et al[24] reported that HA counteracts the fibrinolytic decline induced by TNF-α. Boyce et al[25] indicated that TNF-α down-regulates fibroblastic collagen synthesis within experimental wounds and HA stimulates TNF-α production by human macrophages. In our study, TNF-α mRNA was highly expressed in the PG0.25, 0.5, PL 0.25, and 0.5 groups compared to the RL group. This is in accordance with the results from Abel et al[9], which indicated that beta-glucan induced TNF-α production in wound tissue. In HA group, the level was slightly expressed compared to that of RL group. This is also in accordance with the results from Reijnen et al[24] and Boyce et al[25]. We infer that 0.25% and 0.5% of polysaccharides solutions derived from PG and PL stimulate macrophage activity and increase secretion of uPA, uPAR, and TNF-α by the stimulated macrophage activity. Thus, we conclude that high dose of polysaccharides solutions derived from PG and PL decrease adhesion formation by increasing macrophage activity and enhancing fibrinolytic activity.
In summary, high dose of polysaccharides solutions derived from the fungi, PG and PL are pharmacologic agents that rapidly enhance host resistance to a variety of biological insults through the fibrinolytic system and this involves macrophage activation. In the present study, 0.25 and 0.5% of polysaccharides significantly decreased intraperitoneal adhesion and abscess formation in a rat peritonitis model. It was as effective as HA in the prevention of intraperitoneal adhesion and abscess formation. Additional studies will help elicit whether the use of such polysaccharides and HA in combination may have wider clinical application.
The authors thank J.K. Lee and S.I. Kim for technical assistance. Thanks are also due to Dr. J. Carter, Department of Psychiatry and Behavioural Sciences, University College, London for helping with preparation of manuscript and Dr. S.C. Park, Department of Pharmacology, College of Veterinary Medicine, Kyungpook National University for helping with provision of PG.
Co-correspondents: Kwang-Ho Jang and Hee Kyung Jin
Assistant Editor Guo SY Edited by Gabbe M
| 1. | Ellis H. The cause and prevention of postoperative intraperitoneal adhesions. Surg Gynecol Obstet. 1971;133:497-511. [PubMed] |
| 2. | Menzies D, Ellis H. Intestinal obstruction from adhesions--how big is the problem? Ann R Coll Surg Engl. 1990;72:60-63. [PubMed] |
| 3. | Rotstein OD. Role of fibrin deposition in the pathogenesis of intraabdominal infection. Eur J Clin Microbiol Infect Dis. 1992;11:1064-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Holmdahl L, Ivarsson ML. The role of cytokines, coagulation, and fibrinolysis in peritoneal tissue repair. Eur J Surg. 1999;165:1012-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Sulaiman H, Dawson L, Laurent GJ, Bellingan GJ, Herrick SE. Role of plasminogen activators in peritoneal adhesion formation. Biochem Soc Trans. 2002;30:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Lu HR, Wu Z, Pauwels P, Lijnen HR, Collen D. Comparative thrombolytic properties of tissue-type plasminogen activator (t-PA), single-chain urokinase-type plasminogen activator (u-PA) and K1K2Pu (a t-PA/u-PA chimera) in a combined arterial and venous thrombosis model in the dog. J Am Coll Cardiol. 1992;19:1350-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Bedirli A, Gokahmetoglu S, Sakrak O, Ersoz N, Ayangil D, Esin H. Prevention of intraperitoneal adhesion formation using beta-glucan after ileocolic anastomosis in a rat bacterial peritonitis model. Am J Surg. 2003;185:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Czop JK, Kay J. Isolation and characterization of beta-glucan receptors on human mononuclear phagocytes. J Exp Med. 1991;173:1511-1520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 113] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Abel G, Czop JK. Stimulation of human monocyte beta-glucan receptors by glucan particles induces production of TNF-alpha and IL-1 beta. Int J Immunopharmacol. 1992;14:1363-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Bae JS, Jin HK, Jang KH. The effect of polysaccharides and carboxymethylcellulose combination to prevent intraperitoneal adhesion and abscess formation in a rat peritonitis model. J Vet Med Sci. 2004;66:1205-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Bae JS, Jang KH, Yim H, Park SC, Jin HK. Inhibitory effects of polysaccharides isolated from Phellinus gilvus on benzo(a)pyrene-induced forestomach carcinogenesis in mice. World J Gastroenterol. 2005;11:577-579. [PubMed] |
| 12. | Bucci SJ, Goldstein G, Meinzer FC, Scholz FG, Franco AC, Bustamante M. Functional convergence in hydraulic architecture and water relations of tropical savanna trees: from leaf to whole plant. Tree Physiol. 2004;24:891-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980;29:189-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1078] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 14. | Zühlke HV, Lorenz EM, Straub EM, Savvas V. Pathophysiology and classification of adhesions. Langenbecks Arch Chir Suppl II Verh Dtsch Ges Chir. 1990;345:1009-1016. [PubMed] |
| 15. | Hooker GD, Taylor BM, Driman DK. Prevention of adhesion formation with use of sodium hyaluronate-based bioresorbable membrane in a rat model of ventral hernia repair with polypropylene mesh--a randomized, controlled study. Surgery. 1999;125:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | DeCherney AH, diZerega GS. Clinical problem of intraperitoneal postsurgical adhesion formation following general surgery and the use of adhesion prevention barriers. Surg Clin North Am. 1997;77:671-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 142] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Holmdahl L, Risberg B. Adhesions: prevention and complications in general surgery. Eur J Surg. 1997;163:169-174. [PubMed] |
| 18. | Sulaiman H, Gabella G, Davis MSc C, Mutsaers SE, Boulos P, Laurent GJ, Herrick SE. Presence and distribution of sensory nerve fibers in human peritoneal adhesions. Ann Surg. 2001;234:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL. Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg. 2001;18:260-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 471] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 20. | Parker SJ, Watkins PE. Experimental models of gram-negative sepsis. Br J Surg. 2001;88:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 157] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Suzuki Y, Yamaguchi T. Effects of hyaluronic acid on macrophage phagocytosis and active oxygen release. Agents Actions. 1993;38:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Weigel PH, Fuller GM, LeBoeuf RD. A model for the role of hyaluronic acid and fibrin in the early events during the inflammatory response and wound healing. J Theor Biol. 1986;119:219-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 268] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Reijnen MM, Skrabut EM, Postma VA, Burns JW, van Goor H. Polyanionic polysaccharides reduce intra-abdominal adhesion and abscess formation in a rat peritonitis model. J Surg Res. 2001;101:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Reijnen MM, van Goor H, Falk P, Hedgren M, Holmdahl L. Sodium hyaluronate increases the fibrinolytic response of human peritoneal mesothelial cells exposed to tumor necrosis factor alpha. Arch Surg. 2001;136:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Boyce DE, Thomas A, Hart J, Moore K, Harding K. Hyaluronic acid induces tumour necrosis factor-alpha production by human macrophages in vitro. Br J Plast Surg. 1997;50:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Kim GY, Park HS, Nam BH, Lee SJ, Lee JD. Purification and characterization of acidic proteo-heteroglycan from the fruiting body of Phellinus linteus (Berk. & amp; M.A. Curtis) Teng. Bioresour Technol. 2003;89:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Han SB, Lee CW, Jeon YJ, Hong ND, Yoo ID, Yang KH, Kim HM. The inhibitory effect of polysaccharides isolated from Phellinus linteus on tumor growth and metastasis. Immunopharmacology. 1999;41:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 168] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Lee JH, Cho SM, Song KS, Hong ND, Yoo ID. Characterization of carbohydrate-peptide linkage of acidic heteroglycopeptide with immuno-stimulating activity from mycelium of Phellinus linteus. Chem Pharm Bull (Tokyo). 1996;44:1093-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Kim GY, Roh SI, Park SK, Ahn SC, Oh YH, Lee JD, Park YM. Alleviation of experimental septic shock in mice by acidic polysaccharide isolated from the medicinal mushroom Phellinus linteus. Biol Pharm Bull. 2003;26:1418-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Kim GY, Kim SH, Hwang SY, Kim HY, Park YM, Park SK, Lee MK, Lee SH, Lee TH, Lee JD. Oral administration of proteoglycan isolated from Phellinus linteus in the prevention and treatment of collagen-induced arthritis in mice. Biol Pharm Bull. 2003;26:823-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Jang BS, Kim JC, Bae JS, Rhee MH, Jang KH, Song JC, Kwon OD, Park SC. Extracts of Phellinus gilvus and Phellinus baumii inhibit pulmonary inflammation induced by lipopolysaccharide in rats. Biotechnol Lett. 2004;26:31-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Bae JS, Jang KH, Choi SG, Jo WS, Rhee MH, Park SC. Acute oral toxicity of extract derived from fruiting body of Phellinus gilvus in rats. J Toxicol Pub Health. 2003;19:211-215. |