Published online Feb 14, 2005. doi: 10.3748/wjg.v11.i6.785
Revised: May 28, 2004
Accepted: June 24, 2004
Published online: February 14, 2005
AIM: To investigate synergism of inhibition of telomerase activity and proliferation of human colon cancer cells by combination of telomerase antisense oligonucleotides (ASODNs) simultaneously targeting human telomerase RNA (hTR) and human telomerase reverse transcriptase (hTERT) in vitro.
METHODS: ASODN of hTR and ASODN of hTERT were transfected into human colon cancer SW480 cells by liposomal transfection reagents. Telomerase activity of SW480 cells was examined using telomeric repeat amplification protocol (TRAP)-enzyme-linked immunosorbent assay (PCR-ELISA). Proliferation activity of SW480 cells was tested by methyl thiazolyl tetrazolium assay. Apoptosis and cell cycle were analyzed by flow cytometry.
RESULTS: The telomerase activity and cell survival rate in SW480 cells transfected with 0.2 µmol/L of ASODN of hTR or ASODN of hTERT for 24-72 h were significantly decreased in a time-dependent manner compared with those after treatment with sense oligonucleotides and untreated (telomerase activity: 24 h, 73%, 74% vs 99%, 98%; 48 h, 61%, 55% vs 98%, 99%; 72 h, 41%, 37% vs 99%, 97%; P<0.01; cell survival rate: 24 h, 88%, 86% vs 94%, 98%; 48 h, 49%, 47% vs 94%, 97%; 72 h, 44%, 42% vs 92%, 96%; P<0.01). Moreover, the telomerase activity and the cell survival rate in SW480 cells treated by the combination of telomerase anti-hTR and anti-hTERT were more significantly suppressed than single anti-hTR or anti-hTERT (telomerase activity: 24 h, 59% vs 73%, 74%; 48 h, 43% vs 61%, 55%; 72 h, 18% vs 41%, 37%; P<0.01; cell survival rate: 24 h, 64% vs 88%, 86%; 48 h, 37% vs 49%, 47%; 72 h, 25% vs 44%, 42%; P<0.01). Meanwhile, the apoptosis rates in the combination group were markedly increased compared with those in the single group (24 h, 18.0% vs 7.2%, 7.4%; 48 h, 23.0% vs 13.0%, 14.0%; 72 h, 28.6% vs 13.2%, 13.75; P<0.01). Cells in combination group were arrested at G0/G1 phase.
CONCLUSION: Telomerase anti-hRT and anti-hTERT suppress telomerase activity, and inhibit growth of human colon cancer cells probably via induction of apoptosis and retardation of cell cycle. Additionally, combined use of telomerase ASODNs targeting both hTR and hTERT yields synergistic action selective for human colon cancer.
- Citation: Fu XH, Zhang JS, Zhang N, Zhang YD. Combination of telomerase antisense oligonucleotides simultaneously targeting hTR and hTERT produces synergism of inhibition of telomerase activity and growth in human colon cancer cell line. World J Gastroenterol 2005; 11(6): 785-790
- URL: https://www.wjgnet.com/1007-9327/full/v11/i6/785.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i6.785
Telomerase is a cellular RNA-dependent DNA polymerase that serves to maintain the tandem arrays of telomeric TTAGGG repeats at eukaryotic chromosome ends[1]. Telomeres are highly conserved in organisms ranging from unicellular eukaryocytes to mammals, indicating a strong role of their protective mechanisms in preventing chromosomal ends from undergoing degradation and ligation with other chromosomes. Without telomeric caps human chromosomes would undergo end-to-end fusions, with the formation of dicentric and multicentric chromosomes[1]. These abnormal chromosomes would break during mitosis, resulting in severe damage to the genome and the activation of DNA damage checkpoints, leading to cell senescence or initiation of the apoptotic cell death pathway[2]. Indeed, it has been proposed that telomere length specifies the number of cell divisions a cell can undergo before senescence[3]. Telomerase activity is up-regulated in the vast majority of human tumors, as compared with normal somatic tissues. Expression of the catalytic subunit of telomerase, human telomerase reverse transcriptase (hTERT), in cultured human primary cells reconstitutes telomerase activity and allows immortal growth[4-7]. The rate of telomere DNA shortening is regulated by telomerase expression and activity[8]. Therefore, telomerase inhibitors might be useful as anticancer agents, but there will be an expected log phase between the time when the telomerase is inhibited and the time when the telomere of cancer cells is shortened sufficiently to produce detrimental effects on cell proliferation[9].
Human telomerase mainly consists of three subunits- human telomerase RNA (hTR), human telomerase-associated protein 1 (TP1) and hTERT. Numerous studies have documented that antisense gene therapy directing against telomerase RNA or hTERT component could effectively inhibit telomerase activity and induce apoptosis in gastric cancer, malignant gliomas, colon cancer, and ovarian cancer[10-13]. We hypothesized that simultaneous use of antisense to the RNA portion of hTR, which blocks the template for telomere synthesis[8] and antisense to hTERT, which blocks the promoter of human telomerase catalytic subunit, may provide synergistic antitumor activity against tumor cells that depend on telomerase for telomere maintenance. In this study, we tested the hypothesis that telomerase antisense oligonucleotides (ASODNs) simultaneously targeting hTR and hTERT may provide synergistic antitumor activity against tumor cells that depend on telomerase. ASODNs combination targeting the hTR gene (anti-hTR) and the hTERT gene (anti-hTERT), modified by phosphorothiolation, was transfected into SW480 cells with OligofectamineTM, which has been demonstrated to be telomerase-positive cells. The synergistic action of the combination of anti-hTR and anti-hTERT on telomerase activity, cell survival rates and apoptosis rate in SW480 cells were investigated respectively.
TRAPEZE® ELISA telomerase detection kit. OligofectamineTM reagent was purchased from Invitrogen. RPMI-1640 was obtained from Gibco. Methyl thiazolyl tetrazolium (MTT) and dimethyl sulfoxide (DMSO) were provided by Sigma.
Antisense oligodeoxynucleotides against hTR (anti-hTR) with sequence 5’-CTCAGTTAGGGTTAGAC-3’, which blocks the template for telomere synthesis, and sense oligodeoxynucleotides (SODN) (s-hTR) with sequence 5’-CATTTCTTGCTCTCCACG-3’, antisense oligodeoxy-nucleotides against hTERT (anti-hTERT) with sequence 5’-GGAGCGCGCGGCATCGCGGG-3’, which blocks the promotor for telomere synthesis, and sense oligodeoxy-nucleotides (s-hTERT) with sequence 5’-CCCGCGATGCCGCGCGCTCC-3’, were synthesized by Sangon Biotechnology Engineering Company of Shanghai. The synthesized oligodeoxynucleotides (ODNs) were modified by phosphorothiolation, purified by HPLC, stored at -20 °C.
SW480 cells, a human colon cancer cell line, generously supplied by Department of Biology, Wuhan University, Wuhan, China, were maintained in RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum in a humidified 50 mL/L CO2 atmosphere at 37 °C.
Cultured SW480 cells were divided into seven groups: ASODNs (anti-hTR, anti-hTERT, anti-hTR+anti-hTERT), SODNs (s-hTR, s-hTERT), OligofectamineTM and blank control. The concentration of ASODNs was 0.05 µmol/L, 0.1 µmol/L, 0.2 µmol/L, respectively; the concentration of SODNs was 0.2 µmol/L; OligofectamineTM concentration was varied depending on ODNs dose according to manufacturer’s protocol.
The ODNs transfection was performed with OligofectamineTM according to the manufacturer’s protocol. Briefly, cells were plated into 96-well plates and incubated until the cells reached 30-50% confluency. Before the transfection, ODNs were diluted with serum-free medium. Then, the desired amount of ODNs was incubated for 15-20 min with diluted OligofectamineTM. The ODNs/OligofectamineTM mixture (20 µL) was added drop-wise in 80 µL serum-free RPMI-1640. After incubation for 4 h at 37 °C, 50 µL RPMI-1640 containing 30% fetal bovine serum was added into each well. Cells were analyzed after 24, 48 and 72 h, respectively.
Polymerase chain reaction enzyme-linked immunosorbent assay (PCR-ELISA) was performed according to the manufacturer’s protocol with a minor modification. Cultured SW480 cells were harvested at a density of 1×105 per well and washed with PBS and then homogenized in 200 µL CHAPs lysis buffer and left on ice for 30 min. One hundred and sixty microliters of supernatant was collected after centrifugation (12000 g, 20 min, 4 °C). PCR was performed in 50 µL supernatant containing 10 µL transfer reaction mixture, 2 µL cell extracts added to 48 µL nuclease-free water. The PCR condition is as follows: the telomerase reaction was carried out at 30 °C for 30 min, followed by a two-step PCR amplification (94 °C, 30 s and 55 °C, 30 s for 33 cycles). Five microliters of amplified product and 20 µL denatured reagent were incubated at room temperature, 25 µL hybridization buffer was then added and mixed, and 100 µL of them was distributed in the wells of a microtitering plate. After one hour of incubation (37 °C), 100 µL of anti-DIG-POD working solution was added and incubated for another 30 min followed by the addition of 100 µL TMB substrate solution, and 100 µL of stop reagent was added at last. The absorbance in each well was read at the wavelength of 450 and 690 nm by a microtiter plate reader. The results of A450 min A690>0.80 unit using TSR 8 were judged as a positive control. The negative control was considered as A450 min A690<0.20 unit using lysis buffer. Telomerase activity was considered positive when the value of A450 min A690 of a sample was at least 0.15. Each sample was examined for more than twice.
At the same time, telomeric repeat amplification protocol (TRAP) products were analyzed on a 19% non-denaturing polyacrylamide gel. The gel was stained with silver and analyzed. The internal telomerase assay standard (ITAS) was present in the lane, as described in the kit protocol.
The SW480 cells were seeded onto 96-well plates at the density of 0.5×104 cells per well, and incubated in a humidified atmosphere of 50 mL/L CO2+95% air, until the cells reached 30-50% confluency, then they were transfected with ODNs/OligofectamineTM mixture for 24, 48 and 72 h. After the treatment, 20 µL MTT (5 g/L in PBS) was added. The plates were incubated for 4 h and the blue dye formed was dissolved in 100 µL DMSO. The absorbance at 570 nm was recorded using an ELISA Multiskan reader. Survival rate was calculated as follows: survival rate (%) = (T-B)/(U-B)×100%, where T is the absorbance determined when tumor cells were exposed to drugs; U is the absorbance of untreated cells; B is the absorbance when neither the drug nor MTT was added.
To analyze apoptosis and cell cycle distribution, SW480 cells were trypsinized, harvested with a density of 1×106/L, washed once in ice-cold PBS, fixed and stained with propidium iodide, and then analyzed by flow cytometry.
The data were expressed as mean±SD. Results were analyzed with the software package SPSS 10.0, and one-way ANOVA. P value <0.05 was considered statistically significant.
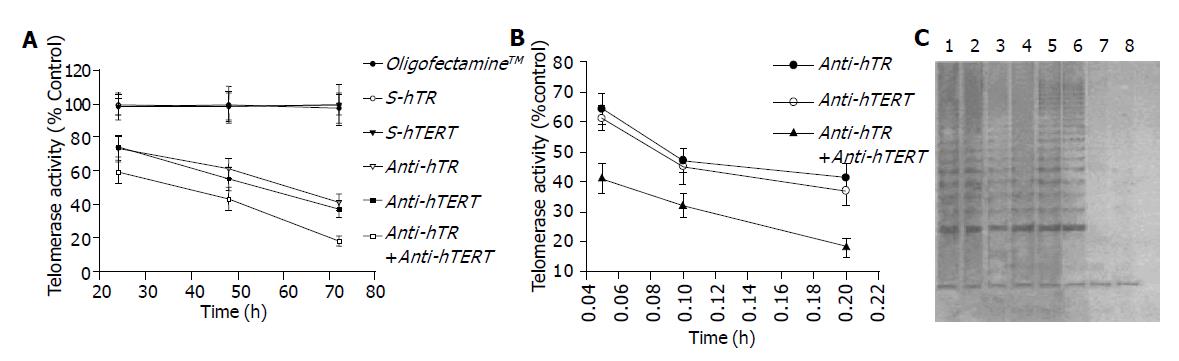
The SW480 cells were transfected with ASODN, SODN and OligofectamineTM and the untreated served as control; telomerase activity of the SW480 cells after transfection of anti-hTR and anti-hTERT was significantly decreased (P<0.01). In addition, after transfection with ASODN of combination of anti-hTR and anti-hTERT, the telomerase activity was significantly lower than that with anti-hTR or anti-hTERT alone (P<0.01). These findings suggested that the combination of anti-hTR and anti-hTERT had synergetic effect on the inhibition of the telomerase activity in human colon cancer cell line (Figure 1A).
Meanwhile, inhibition of anti-hTR or anti-hTERT or both on telomerase activity was in a concentration-dependent manner. The telomerase activity (% control) of SW480 cells transfected with the combination of anti-hTR and anti-hTERT at various concentrations of 0.05, 0.1, 0.2 µmol/L for 72 h decreased from 40.51 to 32.76%, finally to 19.55% (Figure 1B). Lanes shown in PAGE figures support the results (Figure 1C).
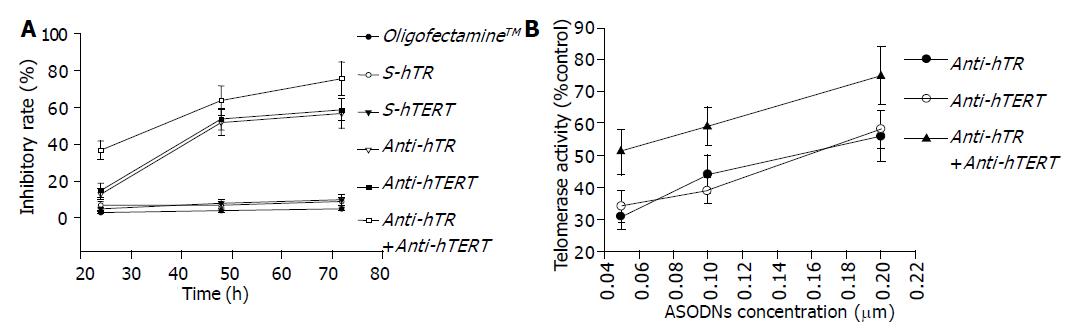
The inhibition on proliferation of SW480 cells was determined after transfection with ASODN, SODN and OligofectamineTM for 24, 48, 72 h, respectively. At 24 h, the survival rates in anti-hTR or anti-hTERT or both groups were significantly decreased compared to those in SODN and OligofectamineTM group (P<0.01). Similarly, combination of anti-hTR and anti-hTERT has synergistic inhibitory effect on cell growth (P<0.01); cell proliferation activity was inhibited in a concentration- and time-dependent manner. The rates of inhibition on proliferation increased from 33.58% at 24 h to 69.34% at 72 h (Figure 2A). Meanwhile, rates of inhibition increased depending on auto-concentration (Figure 2B).
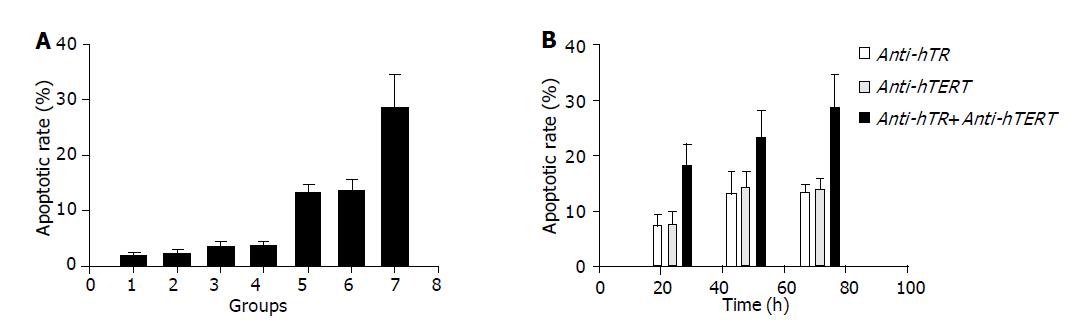
To explore the mechanism contributing to the inhibition of cell growth and the altered cellular morphology, we analyzed the apoptotic rate of SW480 cells treated with anti-hTR or anti-hTERT or both. Compared to cells treated with SODN, OligofectamineTM and untreated control, there was a significant increase in the apoptotic rates of cells treated with anti-hTR and anti-hTERT at 0.2 µmol/L for 72 h (P<0.01). Apoptotic rate of SW480 cells in combination group (28.61%) was markedly higher than that in anti-hTR (13.2%) or anti-hTERT (13.72%) alone (P<0.01) (Figure 3A). Furthermore, as shown in Figure 3B, apoptotic rates gradually increased with treatment time elongation, showing a time-dependent manner.
In SW480 cells treated with combination of anti-hTR and anti-hTERT for 72 h, the cells in G0/G1 increased and in G2/M phases decreased. SW480 cells after treatment with anti-hTR or anti-hTERT alone were accumulated in G0/G1 and G2/M phases, respectively (Table 1).
| Cell cycle distribution (%) | |||
| G0/G1 | S | G2/M | |
| Control | 50.7±2.3 | 36.6±1.8 | 15.5±2.2 |
| OligofectamineTM | 53.8±2.7 | 34.1±3.6 | 12.3±2.6 |
| S-hTR | 53.1±2.8 | 34.4±13.2 | 12.5±1.3 |
| S-hTERT | 50.7±3.5 | 30.1±2.8 | 19.2±2.6 |
| Anti-hTR | 55.0±2.3 | 30.1±2.5 | 14.9±1.9 |
| Anti-hTERT | 49.2±3.4 | 28.8±3.1 | 21.9±2.8b |
| Anti- hTR+anti-hTERT | 63.8±2.3b | 24.9±1.6 | 12.3±2.9 |
Telomerase activity has been detected in 85-90% of various human tumor tissues but is stringently repressed in normal human somatic tissues making telomerase an attractive therapeutic target[14]. Telomerase-associated hTR functions as a template for telomerase elongation by telomerase[15]. hTERT contains reverse transcriptase motifs and functions as the catalytic subunit of telomerase[16]. hTR and hTERT are both attractive targets.
Bachand et al[17] have identified that two independent hTERT binding sites exist within hTR. hTR and hTERT react with each other. Our experiments showed that combination of anti-hTR and anti-hTERT possessed synergistic action on the inhibition of telomerase in the SW480 cell line. This is likely a result of the blocking of both hTR and hTERT components responsible for telomere elongation, thus enhancing the inhibition of telomerase activity.
Telomerase is a ribonucleoprotein DNA polymerase that synthesizes telomeric repeats de novo and is involved in multiple cellular processes, including cell differentiation, proliferation, inhibition of apoptosis, tumorigenesis, and possibly DNA repair and drug resistance[18-21]. Telomerase activity has recently been implicated in the control of the proliferative capacity of normal and malignant cells[22]. In the present study, it has been shown that ASODNs against hTR component and hTERT significantly suppressed SW480 cell growth. In contrast, no significant proliferation inhibition in SW480 cells by sense hTR or sense hTERT was observed. In addition, combination of anti-hTR and anti-hTERT had synergistic inhibitory effects. We suppose that combination of anti-hTR and anti-hTERT augmented the effect on telomerase repression, thus enhanced the inhibitory effect on cell growth.
Some experiments showed that telomerase inhibitors led to cell death probably because progressive telomerase shortening was responsible for decreased cell proliferation. However, a number of studies have documented that telomerase inhibitors resulted in proliferation inhibition likely via induction of apoptosis[23]. Kondo et al[24] demonstrated that treatment with antisense telomerase inhibited telomerase activity and subsequently induced either apoptosis or differentiation. Regulation of these two distinct pathways may be dependent on the expression of interleukin-1 beta-converting enzyme (ICE) or cyclin-dependent kinase inhibitors (CDKIs). In the present study, it showed that anti-hTR and anti-hTERT induced apoptosis in SW480 cells. Apoptotic rates in combination group were markedly increased than that in the single group. It was established that anti-hTR and anti-hTERT inhibited the growth of SW480 cells through the induction of apoptosis. Combination of anti-hTR and anti-hTERT has synergistic induction of apoptosis.
Some human tumor cells without any telomerase activity are able to maintain the length of their telomeres, indicating the existence of one or more non-telomerase mechanisms for telomere maintenance which have been termed alternative lengthening of telomeres (ALT)[25,26]. ALT has been detected in a variety of human tumors, including sarcomas, glioblastomas, and cancers of the lung, kidney, adrenal, breast, and ovary[27]. An implication of the existence of ALT is that tumors using this telomere maintenance mechanism (including mixed telomerase-positive/ALT-positive tumors) will be resistant to telomerase inhibitors. Also, telomerase inhibitors will put tumors that are initially telomerase-positive under strong selection pressure for activation of ALT. Therefore, combination therapy using ALT and telomerase inhibitors may help prevent the emergence of drug resistance. A novel telomerase inhibitor, telomestatin, is a clinical candidate as a dual inhibitor of ALT and telomerase[28]. Lee et al[29] reported that neoplastic cells from telomerase RNA null mice (mTERC-/-) showed enhanced chemosensitivity to doxorubicin or related double strand DNA break-inducing agents. Telomere dysfunction, rather than telomere inhibition, is proposed to be the principal determinant governing chemosensitivity specifically to double strand DNA break-inducing agents[29]. In this study, we detected apoptosis in tumor cells just 24 h after the combination of anti-hTR and anti-hTERT treatment in a dose-dependent manner. It is likely to raise the possibility that antitumor effect of the combination of anti-hTR and anti-hTERT occurs through the following two pathways: (1) A short-term effect on apoptosis was induced rapidly by the combination of anti-hTR and anti-hTERT. (2) A long-term inhibitory effect on telomerase activity was provoked, and cell death was caused when telomere length was critically shortened by telomeric DNA.
Zhu et al[30] have reported that telomerase activity was likely to be regulated in a cell cycle-dependent manner. As the cell progresses through the cell cycle, telomerase activity gradually increased through the G1/S phase, reached its maximum level in S phase, and decreased through the G2/M phase. These observations may be consistent with the present experimental results showing that inhibition of telomerase by the combination of anti-hTR and anti-hTERT led to increased G0/G1 phase cells. Although SW480 cells treated with anti-hTERT were arrested in G2/M phase, we speculated that combination of anti-hTR and anti-hTERT augmented retardation, causing cells to accumulate in G0/G1 phase.
In conclusion, in this study, it has been shown that anti-hTR and anti-hTERT have specific inhibition on SW480 cells. There is no significant difference between anti-hTR and anti-hTERT, but the combination of anti-hTR and anti-hTERT has significant synergistic action. Our data suggest that the strategy of telomerase ASODNs simultaneously targeting hTR and hTERT may offer a potential antitumor tool against human colon cancer and other telomerase-positive tumors.
Edited by Zhu LH
| 1. | van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell. 1998;92:401-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1285] [Cited by in RCA: 1302] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 2. | de Lange T, Jacks T. For better or worse? Telomerase inhibition and cancer. Cell. 1999;98:273-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Harley CB. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991;256:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 782] [Cited by in RCA: 773] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 4. | Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3573] [Cited by in RCA: 3446] [Article Influence: 127.6] [Reference Citation Analysis (0)] |
| 5. | Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 918] [Cited by in RCA: 903] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 6. | Jiang XR, Jimenez G, Chang E, Frolkis M, Kusler B, Sage M, Beeche M, Bodnar AG, Wahl GM, Tlsty TD. Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat Genet. 1999;21:111-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 470] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 7. | Morales CP, Holt SE, Ouellette M, Kaur KJ, Yan Y, Wilson KS, White MA, Wright WE, Shay JW. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat Genet. 1999;21:115-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 554] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 8. | Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J. The RNA component of human telomerase. Science. 1995;269:1236-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1606] [Cited by in RCA: 1606] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 9. | White LK, Wright WE, Shay JW. Telomerase inhibitors. Trends Biotechnol. 2001;19:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 131] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Naka K, Yokozaki H, Yasui W, Tahara H, Tahara E, Tahara E. Effect of antisense human telomerase RNA transfection on the growth of human gastric cancer cell lines. Biochem Biophys Res Commun. 1999;255:753-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Mukai S, Kondo Y, Koga S, Komata T, Barna BP, Kondo S. 2-5A antisense telomerase RNA therapy for intracranial malignant gliomas. Cancer Res. 2000;60:4461-4467. [PubMed] |
| 12. | Jiang YA, Luo HS, Fan LF, Jiang CQ, Chen WJ. Effect of antisense oligodeoxynucleotide of telomerase RNA on telomerase activity and cell apoptosis in human colon cancer. World J Gastroenterol. 2004;10:443-445. [PubMed] |
| 13. | Yuan Z, Mei HD. Inhibition of telomerase activity with hTERT antisense increases the effect of CDDP-induced apoptosis in myeloid leukemia. Hematol J. 2002;3:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5156] [Cited by in RCA: 5234] [Article Influence: 168.8] [Reference Citation Analysis (0)] |
| 15. | Lingner J, Hughes TR, Shevchenko A, Mann M, Lundblad V, Cech TR. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science. 1997;276:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 892] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 16. | Kurreck J. Antisense technologies. Improvement through novel chemical modifications. Eur J Biochem. 2003;270:1628-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 752] [Cited by in RCA: 722] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 17. | Bachand F, Autexier C. Functional regions of human telomerase reverse transcriptase and human telomerase RNA required for telomerase activity and RNA-protein interactions. Mol Cell Biol. 2001;21:1888-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Urquidi V, Tarin D, Goodison S. Role of telomerase in cell senescence and oncogenesis. Annu Rev Med. 2000;51:65-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Fu W, Begley JG, Killen MW, Mattson MP. Anti-apoptotic role of telomerase in pheochromocytoma cells. J Biol Chem. 1999;274:7264-7271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 182] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Nugent CI, Bosco G, Ross LO, Evans SK, Salinger AP, Moore JK, Haber JE, Lundblad V. Telomere maintenance is dependent on activities required for end repair of double-strand breaks. Curr Biol. 1998;8:657-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 299] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 21. | Ishikawa T, Kamiyama M, Hisatomi H, Ichikawa Y, Momiyama N, Hamaguchi Y, Hasegawa S, Narita T, Shimada H. Telomerase enzyme activity and RNA expression in adriamycin-resistant human breast carcinoma MCF-7 cells. Cancer Lett. 1999;141:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Greider WA. A laser for hard and soft tissue applications. Dent Today. 1998;17:68-70, 72-73. [PubMed] |
| 23. | Wong SC, Lo ES, Chan AK, Lee KC, Hsiao WL. Nuclear beta catenin as a potential prognostic and diagnostic marker in patients with colorectal cancer from Hong Kong. Mol Pathol. 2003;56:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Kondo S, Tanaka Y, Kondo Y, Hitomi M, Barnett GH, Ishizaka Y, Liu J, Haqqi T, Nishiyama A, Villeponteau B. Antisense telomerase treatment: induction of two distinct pathways, apoptosis and differentiation. FASEB J. 1998;12:801-811. [PubMed] |
| 25. | Bryan TM, Englezou A, Gupta J, Bacchetti S, Reddel RR. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 1995;14:4240-4248. [PubMed] |
| 26. | Dunham MA, Neumann AA, Fasching CL, Reddel RR. Telomere maintenance by recombination in human cells. Nat Genet. 2000;26:447-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 644] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 27. | Henson JD, Neumann AA, Yeager TR, Reddel RR. Alternative lengthening of telomeres in mammalian cells. Oncogene. 2002;21:598-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 473] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 28. | Shin-ya K, Wierzba K, Matsuo K, Ohtani T, Yamada Y, Furihata K, Hayakawa Y, Seto H. Telomestatin, a novel telomerase inhibitor from Streptomyces anulatus. J Am Chem Soc. 2001;123:1262-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 392] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 29. | Lee KH, Rudolph KL, Ju YJ, Greenberg RA, Cannizzaro L, Chin L, Weiler SR, DePinho RA. Telomere dysfunction alters the chemotherapeutic profile of transformed cells. Proc Natl Acad Sci USA. 2001;98:3381-3386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Zhu X, Kumar R, Mandal M, Sharma N, Sharma HW, Dhingra U, Sokoloski JA, Hsiao R, Narayanan R. Cell cycle-dependent modulation of telomerase activity in tumor cells. Proc Natl Acad Sci USA. 1996;93:6091-6095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 164] [Article Influence: 5.7] [Reference Citation Analysis (0)] |