Published online Feb 7, 2005. doi: 10.3748/wjg.v11.i5.741
Revised: April 25, 2004
Accepted: May 9, 2004
Published online: February 7, 2005
AIM: To investigate the expression of vascular endothelial growth factor (VEGF) and microvascular density (MVD) count in pediatric malignant liver tumor and their clinical significances.
METHODS: Fourteen children with malignant liver tumors including seven hepatocellular carcinomas (HCCs), five hepatoblastomas, one malignant mesenchymoma and one rhabdomyosarcoma were studied. Twelve adult HCC samples served as control group. All samples were examined with streptavidin-biotin peroxidase (SP) immunohistochemical staining for VEGF expression and MVD count.
RESULTS: VEGF positive expression in all pediatric malignant liver tumors was significantly higher than that in adult HCC (0.4971±0.14 vs 0.4027±0.03, P<0.05). VEGF expression in pediatric HCC group was also markedly higher than that in adult HCC group (0.5665±0.10 vs 0.4027±0.03, P<0.01) and pediatric non-HCC group (0.5665±0.10 vs 0.4276±0.15, P<0.05). The mean value of MVD in pediatric malignant liver tumors was significantly higher than that in adult HCC (33.66±12.24 vs 26.52±4.38, P<0.05). Furthermore, MVD in pediatric HCC group was significantly higher compared to that in adult HCC group (36.94±9.28 vs 26.52±4.38, P<0.05), but there was no significant difference compared to the pediatric non-HCC group (36.94±9.28 vs 30.37±14.61, P>0.05). All 7 children in HCC group died within 2 years, whereas the prognosis in pediatric non-HCC group was better, in which two patients survived more than 5 years.
CONCLUSION: Children with malignant liver tumors, especially with HCC, may have extensive angiogenesis that induces a rapid tumor growth and leads to a poor prognosis.
- Citation: Sun XY, Wu ZD, Liao XF, Yuan JY. Tumor angiogenesis and its clinical significance in pediatric malignant liver tumor. World J Gastroenterol 2005; 11(5): 741-743
- URL: https://www.wjgnet.com/1007-9327/full/v11/i5/741.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i5.741
We reported a group of 26 children with primary liver malignant tumor during a ten year period, in which hepatocellular carcinoma (HCC) was in the majority[1]. These patients had a very poor prognosis due to frequent metastases and rapid recurrence, and died in a short time no matter what kind of therapeutic procedures was taken. The characteristics of pediatric malignant liver tumor, especially HCC, are worthwhile to be studied. Recently, the roles of vascular endothelial growth factor (VEGF) in the growth and metastasis of tumors have drawn extensive attention. We presume there is an unusual angiogenesis that might be related with VEGF expression in pediatric liver malignant tumor. In this study, we investigated the VEGF expression and microvascular density (MVD) in primary liver malignant tumor in children, and elucidated the possible reasons and correlative factors responsible for the high malignant behaviors of these tumors.
Among 26 children with malignant liver tumor who were treated during 1992 to 2001, 14 cases (10 boys and 4 girls with an average age of 68.6 mo and range 0.8-182 mo) underwent surgery and were pathologically diagnosed as HCC (7 cases, 50%), hepatoblastoma (HB, 5 cases, 35.7%), malignant mesenchymoma (1 case, 7.14%) and rhabdomyosarcoma (1 case, 7.14%). In HCC group, 4 had multiple tumors and 3 had a single tumor in liver, but the patients with HB, malignant mesenchymoma or rhabdomyosarcoma had only single tumor in liver. Alpha-fetoprotein (AFP) and HBsAg in all patients were measured before operation.
The specimens obtained from normal liver, tumor and tumor surrounding tissues were fixed in 100 mL/L formalin, embedded in paraffin and serially sectioned (4 μm thick). Routine histopathologic evaluation of the tumor was performed using hematoxylin and eosin staining, streptavidin-biotin peroxidase (SPTM kit, ZYMED, USA) procedure was used for immunohisto-chemical staining of VEGF and MVD. The first antibodies were rabbit anti-human VEGF polyclonal antibody (1:20, Santa Cruz Biotechnology, Inc) and mouse anti-human CD34 (endothelial cell marker for measuring MVD) monoclonal antibody (1:50, NeoMarkers), respectively. Another 12 specimens obtained from adult HCC (ranging in age from 36 to 59 years) were used as control. All steps of manipulation were carried out according to the manufacturer’s instructions of the kit. Positive VEGF staining appeared as brown in cytoplasm was analyzed with microscopy and computer-image system (HPIAS-1000, QianPing Co.). The areas of positively stained cells in five visual fields were measured by HPIAS-1000 in each section. According to the criteria of Weidner et al[2], the number of capillaries and microvessels which were endothelial-lined within tumor was measured by MVD count. The distribution of microvessels in each section was observed primarily under a low-power microscope, and then 5 fields with abundant microvessels were selected for counting under a high power microscope (400 magnification), and a mean value was calculated.
The survival time of all children in HCC and non-HCC groups was calculated until their death or September of 2003, and described with the Kaplan-Meier curve.
The values of VEGF positive expression and MVD count were presented as mean±SD. Student’s t test was used to analyse the data. P<0.05 was considered statistically significant.
In children with HCC, HB and two cases of malignant mesenchymoma or rhabdomyosarcoma, the positive rate of AFP was 71.2% (5/7), 100% (5/5) and 0%, respectively. The positive rate of HBsAg was 43% (3/7), 0% and 0%, respectively.
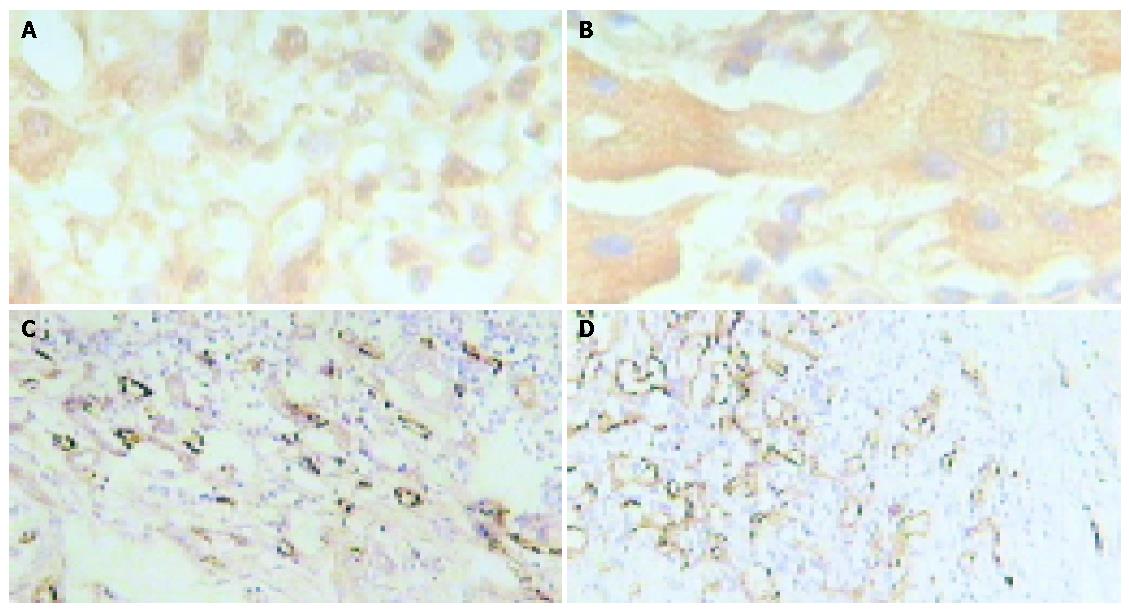
VEGF positive expression was brown in sections of tumor tissue (Figures 1A, B). It was significantly higher in all pediatric malignant liver tumors than in adult HCC (0.4971±0.14 vs 0.4027±0.03, P<0.05). VEGF expression in pediatric HCC group was also markedly higher than that in adult HCC group (0.5665±0.10 vs 0.4027±0.03, P<0.01) and pediatric non-HCC group (0.5665±0.10 vs 0.4276±0.15, P<0.05, Table 1). The distribution of microvessels was asymmetric in tumor tissue and the densest distribution was in the edge of the tumor (Figures 1C, D). The mean value of MVD in pediatric malignant liver tumor was significantly higher than that in adult HCC (33.66±12.24 vs 26.52±4.38, P<0.05, Table 1). Furthermore, MVD in pediatric HCC group was markedly higher compared to the adult HCC group (36.94±9.28 vs 26.52±4.38, P<0.05), but there was no significant difference compared to the pediatric non-HCC group (36.94±9.28 vs 30.37±14.61, P>0.05, Table1).
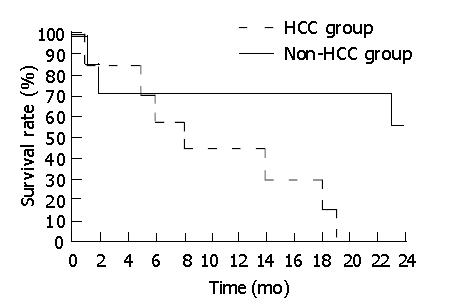
All patients were followed-up. All children in HCC group died within two years after operation. The patients in non-HCC group had a better prognosis. Among the survivors, two cases survived for over 5 years. Kaplan-Meier curve showed that although all patients underwent surgery procedures, there was a significant difference in survival rate between patients with HCC and without HCC, the survival rate of non-HCC group was notably higher than that of HCC group (Figure 2).
In this study, the clinical characteristics of pediatric primary malignant liver tumor were as follows: (1) HCC cases out numbered HB cases. This result was different from previous reports that the incidence of HB was the highest in pediatric malignant liver tumors[3]. (2) HBsAg was positive in 43%(3/7) of all HCC cases, and negative in all HB cases, implying that hepatitis B virus (HBV) infection might be responsible for HCC in children. (3) AFP was positive in all HB and most HCC cases, but HBsAg and AFP were negative in patients with mesenchymoma or rhabdomyosarcoma. (4) The resection rate of pediatric malignant liver tumor was low, accounting for 38.5% (10/26) during the same period, and the resection rate of HCC was only 21.05% in this study.
Clinical results have shown pediatric malignant liver tumors grow rapidly and are often at the late stage when found or diagnosed. Furthermore, pediatric malignant liver tumors, especially HCC, have a very poor prognosis even if treated. In pediatric HCC group of our study, none survived for over two years. This poor prognosis might be due to the rapid recurrence and metastasis of pediatric malignant liver tumor even after surgical resection.
A more recent research indicates that the growth and metastasis of solid tumors are dependent on the formation of new blood vessels[4]. The onset of angiogenesis is believed to be an early event in tumorigenesis and may facilitate tumor progression and metastasis[5]. Several growth factors with angiogenic activity have been described, including fibroblast growth factor (FGF), platelet derived growth factor (PDGF) and VEGF. Among the angiogenesis factors, VEGF is the most important one and a hot spot of study at present. It has been reported that VEGF is a dimeric glycoprotein with a structural homology with PDGF[6,7], and also a highly specific mitogen for blood vessel endothelial cells, which can stimulate endothelial cells of microvessels to proliferate and increase permeability, resulting in tumor angiogenesis[8]. On the other hand, tumor MVD is not only the criterion for evaluating angiogenesis, but also has a close relation with malignant behaviors of tumor, such as recurrence and metastasis[9]. The higher the density of the new blood vessels is in tumor, the worse the prognosis of patients is. Thus VEGF and MVD were chosen to measure and understand the angiogenesis event in pediatric liver malignant tumor in this study.
The VEGF expression and MVD count in pediatric malignant liver tumor, especially in pediatric HCC group, were significantly higher than those in adult HCC group. The results indicate there is stronger angiogenesis in pediatric liver malignant tumor which might be an important reason why tumor growth is faster and prognosis is poorer in children than in adults. Among the children with malignant liver tumor, VEGF positive expression and MVD count in HCC group were also higher than those in non-HCC group. On the other hand, there were also significant differences in survival rate between HCC and non-HCC groups. Almost all patients in HCC group died within two years after operation, but more patients in non-HCC group survived for over two years. Two possibilities might be accountable for this outcome. First, since this study consisted of a small number of patients, the cases were not sufficient to reflect the different characteristics of angiogenesis of pediatric HCC and non-HCC. Second, except for angiogenesis, other factors, such as types of tumor, differentiation degree, course of disease, location and metastasis, might be responsible for the prognosis. In the non-HCC group, two patients with malignant mesenchymoma or rhabdomyosarcoma are still alive. Interestingly, although without statistical significance, their VEGF expression was negative.
The strong angiogenesis features in pediatric malignant liver tumor can predict the rapid growth, easy metastasis and recurrence, and extremely poor prognosis after operation. All these findings indicate that antiangiogenic therapy could prolong the survival time of malignant liver tumor patients after operation. The cause of the strong expression of VEGF and increased MVD in malignant liver tumor in children is still unclear, thus further studies are needed.
Edited by Wang XL and Kumar M Proofread by Zhu LH
| 1. | Sun XY, Yuan JY, Chen XP. Diagnosis and treatment for liver malignant tumor in children. J Hepatobiliary Surg. 2000;8:180-182. |
| 2. | Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1277] [Cited by in RCA: 1300] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 3. | Chen JC, Chen CC, Chen WJ, Lai HS, Hung WT, Lee PH. Hepatocellular carcinoma in children: clinical review and comparison with adult cases. J Pediatr Surg. 1998;33:1350-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 62] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Du JR, Jiang Y, Zhang YM, Fu H. Vascular endothelial growth factor and microvascular density in esophageal and gastric carcinomas. World J Gastroenterol. 2003;9:1604-1606. [PubMed] |
| 5. | Folkman J, Watson K, Ingber D, Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989;339:58-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1358] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 6. | Ferrara N, Houck KA, Jakeman LB, Winer J, Leung DW. The vascular endothelial growth factor family of polypeptides. J Cell Biochem. 1991;47:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 387] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1572] [Cited by in RCA: 1588] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 8. | Bussolino F, Mantovani A, Persico G. Molecular mechanisms of blood vessel formation. Trends Biochem Sci. 1997;22:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 339] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 9. | Aceñero MJ, González JF, Gallego MG, Ballesteros PA. Vascular enumeration as a significant prognosticator for invasive breast carcinoma. J Clin Oncol. 1998;16:1684-1688. [PubMed] |