Published online Feb 7, 2005. doi: 10.3748/wjg.v11.i5.737
Revised: February 5, 2004
Accepted: February 26, 2004
Published online: February 7, 2005
AIM: To investigate the effects of psychological stress on small intestinal motility and expression of cholecystokinin (CCK) and vasoactive intestinal polypeptide (VIP) in plasma and small intestine, and to explore the relationship between small intestinal motor disorders and gastrointestinal hormones under psychological stress.
METHODS: Thirty-six mice were randomly divided into psychological stress group and control group. A mouse model with psychological stress was established by housing the mice with a hungry cat in separate layers of a two-layer cage. A semi-solid colored marker (carbon-ink) was used for monitoring small intestinal transit. CCK and VIP levels in plasma and small intestine in mice were measured by radioimmunoassay (RIA).
RESULTS: Small intestinal transit was inhibited (52.18±19.15% vs 70.19±17.79%, P<0.01) in mice after psychological stress, compared to the controls. Small intestinal CCK levels in psychological stress mice were significantly lower than those in the control group (0.75±0.53 μg/g vs 1.98±1.17 μg/g, P<0.01), whereas plasma CCK concentrations were not different between the groups. VIP levels in small intestine were significantly higher in psychological stress mice than those in the control group (8.45±1.09 μg/g vs 7.03±2.36 μg/g, P<0.01), while there was no significant difference in plasma VIP levels between the two groups.
CONCLUSION: Psychological stress inhibits the small intestinal transit, probably by down-regulating CCK and up-regulating VIP expression in small intestine.
- Citation: Cao SG, Wu WC, Han Z, Wang MY. Effects of psychological stress on small intestinal motility and expression of cholecystokinin and vasoactive intestinal polypeptide in plasma and small intestine in mice. World J Gastroenterol 2005; 11(5): 737-740
- URL: https://www.wjgnet.com/1007-9327/full/v11/i5/737.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i5.737
In recent years, with rapid development of society and increasing competition pressure, psychological stress, which originates in society has imposed impacts on human health, and has become an important stressor. Psychological stress is widely believed to play a major role in gastrointestinal motor disorders, especially in irritable bowel syndrome (IBS) and functional dyspepsia (FD), by precipitating exacerbation of symptoms[1,2]. Available data clearly demonstrate that in experimental animals the most consistent patterns of gastrointestinal motor alternations induced by psychological stress are delayed gastric emptying[3,4] and accelerated colon transit[5,6]. However, almost no valid data are available on small intestinal motility disorders. Previous studies of psychological stress[7-9] (cold stress, restraint stress, foot-shock stress and swim stress), were all focused on somatic stress. In common, both central and peripheral nervous pathways are involved in the release of gastrointestinal hormones due to psychological stress, thus modulating gastrointestinal motility[10]. A large body of evidence derived from experiments suggest that CCK could accelerate small intestinal transit[11,12], while VIP could inhibit it[13]. However, there are few studies on whether gut hormones are involved in modulating small intestinal motility under psychological stress.
In the present study, experimental animals were obtained to test the relationship between small intestinal motility and release of gastrointestinal hormones during psychological stress. Furthermore, small intestinal motility disorders in response to CCK and VIP due to psychological stress were studied.
Thirty-six healthy male mice (provided by Qinglongshan Experimental Animal Center) weighing 20-30 g were used in this study. Mice were housed individually in cages at constant room temperature with a 12-h light/dark cycle and had free access to laboratory chow and water. CCK kit was purchased from Neurobiological Department of Second Military Medical University. VIP kit was purchased from Beijing Haikerui Biological Technology Center.
Thirty-six mice were randomly divided into psychological stress group and control group. Each contained 18 mice. Mice in psychological stress group were housed in the bottom of two-layer cage, with a hungry cat housed in the upper layer of the cage for 10 min each day for 10 d, but mice and the cat had no physical contact[14]. Procedure for the control group mice were the same as psychological stress group except for no contact with the cat.
The carbon-ink transit test was modified as described. Mice were deprived of food for 24 h and water for 12 h prior to experiment, and 0.25 mL carbon-ink was administered into their stomachs by orogastric gavage. Twenty-five minutes later, the mice were killed, abdomen was opened and small intestine was dissected. The total length of the small intestine (pylorus-cecum) and the distance traveled by carbon-ink were measured. Results were expressed as ratio (percentage) of the distance traveled by carbon-ink to the total length of the small intestine. Small intestine was washed with normal saline after measuring. Water was absorbed by filter paper and small intestine was kept in a dry bottle.
Immediately after the mice were killed, blood samples were collected into chilled tubes containing 0.3 μL ethylenediamine tetraacetic acid (EDTA) and 1000 KIU aprotinin. The blood was centrifuged at 3000 r/min at 4 °C for 10 min. The plasma was stored at -70 °C until assayed.
Samples of small intestine were placed in saline and boiled for 10 min. Water was absorbed by filter paper. Small intestine was weighed by analytical balance and then dissolved in 1 mmol/L ice-cold acetic acid (0.3 mL/100 g), homogenized and the same volume 1 mmol/L sodium hydroxide was added and then they were left at room temperature for 20 min, and centrifuged at 3000 r/min at 4 °C for 10 min. The supernatants were stored at -70 °C until assayed.
Plasma and small intestine CCK levels were measured using radioimmunoassay (RIA). Briefly, samples and standards diluted in the perfusion medium were incubated with CCK antiserum in tubes for 24 h at 4 °C. After addition of 125I-CCK, all samples were further incubated for 24 h at 4 °C to reach equilibrium. Antibody-bound and free tracers were separated by addition of 0.4 mL of activated charcoal.
VIP was determined by radioimmunoassay. Samples and standards diluted in the perfusion medium were incubated with VIP antiserum in tubes for 48 h at 4 °C. After addition of 125I labeled VIP, all samples were further incubated for 48 h at 4 °C. After incubation, antibody-bound and free tracers were separated by addition of 0.4 mL of activated charcoal.
Data were expressed as mean±SD. Experimental results were analyzed by t test, P<0.05 was considered statistically significant.
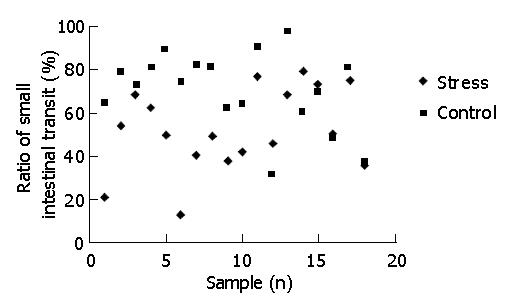
After 25 min of intragastric carbon-ink administration, the overall mean ratio of small intestinal transit (P<0.01) under psychological stress was lower than that of the control (52.18±19.15% vs 70.19±17.79%), indicating that psychological stress could inhibit small intestinal transit. Figure 1 presents individual data for the ratio of small intestinal transit (percentage of the distance traveled by carbon-ink to the total length of the small intestine).
CCK levels in small intestine of experimental psychological stress mice were significantly lower than those of the control (0.75±0.53 μg/g vs 1.98±1.17 μg/g, P<0.01). However, no significant changes in plasma CCK levels were observed in experimental psychological stress mice compared to the control (53.88±33.17 ng/L vs 52.70±20.10 ng/L, P>0.05) (Table 1).
| Group | No. of animals | Small intestine mg/g) | Plasma (ng/L) |
| Control | 18 | 1.98±1.17 | 52.70±20.10 |
| Stress | 18 | 0.75±0.53b | 53.88±33.17 |
VIP levels in small intestine of experimental psychological stress mice were significantly higher than those of the control (8.45±1.09 μg/L vs 7.03±2.36 μg/L, bP<0.01). However, plasma VIP levels showed no significant difference between the two groups (201.58±103.23 μg/L vs 190.05±90.08 μg/L, P>0.05)(Table 2).
The association between psychological stress and small intestinal motility has been postulated for about twenty years. Some studies in experimental animals indicate contradictory results, and the mechanism by which psychological stress affects small intestinal motility is not well understood. Ditto et al[15] recently reported that stress enhanced small intestinal transit and produced a statistically significant reduction in the mean small intestinal transit time. However, Tsukada et al[16] demonstrated that psychological stress induced inhibition of small intestinal transit. In the present study, our results were similar to Tuskada’s. After 25 min of intragastric carbon-ink administration, the ratio of small intestinal transit significantly decreased during psychological stress. Previous studies show that patients with gastrointestinal motility disorders, especially irritable bowel syndrome (IBS), symptoms of abdominal pain and bloating are precipitated after psychological stress (life events or trauma). The mechanism may be that psychological stress induces decrease in small intestinal transit. Intestinal gas could not be evacuated effectively leading to gas retention, which evokes a series of symptoms, especially pain and bloating[17].
In this study, we showed that CCK levels in small intestine of psychological stress mice were significantly lower than those of the controls, while they did not alter in plasma. CCK, a peptide secreted from I cells of gastrointestine responds to the presence of nutrients in the small intestine. It is also synthesized in brain[18]. Two CCK receptor subtypes that differ markedly in their pharmacological profiles and anatomical distribution have been identified and characterized[19]. CCK-A receptors exist predominantly at the peripheral level where they are responsible for the digestive effects of CCK[20]. However, high densities of brain CCK-B receptors are present in cortical and limbic blood. Endogenous CCK appears to act in part by paracrine and endocrine mechanisms to affect gut motility[21]. It is well established that endogenous CCK regulates gastrointestinal motility, including delay of gastric emptying[22], acceleration of small intestinal transit[23] and enhancement of colon motor[24]. Various studies have provided evidence for the mechanism of CCK underlying small intestinal motility through involvement of CCK-A receptors in small intestine. The selective CCK-A receptor antagonists (devazepide and lorglumide) effectively attenuate this effect while CCK-B receptor antagonists have no effect on it[25]. Furthermore, both afferent and efferent nerves are implicated in CCK motor actions in the gastrointestinal tract. Patterson et al[27] performed immunohistochemistry using an antibody directed to the C-terminal region of the CCK-A receptor, and found that intestinal cells of Cajal (ICC) in the sphincter muscle and the circular muscle of proximal duodenum displayed strong CCK-A receptor immunoreactivity; at the same time, selection neurons in the myenteric ganglia and a few of single neurons in the submucosa near the proximal duodenum, also expressed strong CCK-A receptor immunoreactivity. In view of these results, we can conclude that the effect of psychological stress on intestinal motility and transit may be partially mediated by decreasing intraluminal concentrations of CCK, which leads to reduced migrating myoelectric complex (MMC) activity of the small intestine, prolonging MMC cycle length, reducing contraction frequency and decreaseing propagation velocity.
VIP, which is released from D1 cells of gut through exocrine into the lumen, has an established role in the regulation of gastrointestinal motor function. VIP is widely distributed in gut. In addition to GI mucosa[28], it is also found in submucous and myenteric plexus, central and peripheral nervous systems[29,30]. Furthermore, a large number of VIP receptors are expressed in those regions[31-33]. Sayadi et al[34] analyzed the distribution of VIP receptors using autoradiographic techniques, and found that the density of VIP receptors was greatest in duodenal mucosa but was lower in jejunal and ileal mucosa. Moreover, previous studies have provided evidence that VIP acts via two receptors (VIP1 and VIP2). In the intestine, VIP1 receptor is significantly higher than VIP2 receptor[35]. A great number of data demonstrate that VIP has various physiologic functions on small intestinal motility. First, VIP exerts direct stimulatory effect on intestinal smooth muscle, reduces the percentage motor index of pressure waves. This effect of VIP could be partially antagonized by VIP receptor antagonists[36]. Second, intraduodenal infusion of VIP in rats disrupts the MMC, prolongs MMC cycle length and decreases propagation velocity, while VIP receptor antagonists could reverse this effect[37-39]. Third, the effect of VIP on intestinal motility involves both myenteric and submucosal neurons, resulting in increased contraction of longitudinal smooth muscle[40]. Naslund et al[41] demonstrated that intraduodenal but not plasma concentrations of VIP showed an association with the MMC. In the present study, we showed that VIP levels in small intestine were significantly higher than those of the control, but had no difference in plasma. It indicates that small intestine VIP plays an important role in the inhibition of small intestinal transit caused by psychological stress.
The results of this study suggest that gastrointestinal motility disorders during psychological stress may be partially mediated by release of gut hormones from small intestine. But the following questions need to be answered in future: how does psychological stress modulate gut hormone release from small intestine and how are gastrointestinal motility disorders caused by gut hormones in small intestine.
In summary, psychological stress does induce changes in the small intestinal motility. Changes of CCK and VIP levels in the small intestine of mice may be closely related with the inhibition of small intestine transit. However, there is no relationship between gastrointestinal dysmotility and gut hormones in plasma during psychological stress.
Edited by Wang XL and Zhu LH
b
| 1. | Mayer EA, Naliboff BD, Chang L, Coutinho SV. V. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2001;280:G519-G524. [PubMed] |
| 2. | Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. J Clin Psychiatry. 2001;62 Suppl 8:28-36; discussion 37. [PubMed] |
| 3. | Tsukada F, Nagura Y, Abe S, Sato N, Ohkubo Y. Effect of restraint and footshock stress and norepinephrine treatment on gastric emptying in rats. Biol Pharm Bull. 2003;26:368-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Mistiaen W, Blockx P, Van Hee R, Bortier H, Harrisson F. The effect of stress on gastric emptying rate measured with a radionuclide tracer. Hepatogastroenterology. 2002;49:1457-1460. [PubMed] |
| 5. | Martínez V, Taché Y. Role of CRF receptor 1 in central CRF-induced stimulation of colonic propulsion in rats. Brain Res. 2001;893:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Maillot C, Million M, Wei JY, Gauthier A, Taché Y. Peripheral corticotropin-releasing factor and stress-stimulated colonic motor activity involve type 1 receptor in rats. Gastroenterology. 2000;119:1569-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 164] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Tsukada F, Sugawara M, Kohno H, Ohkubo Y. Evaluation of the effects of restraint and footshock stress on small intestinal motility by an improved method using a radionuclide, 51Cr, in the rat. Biol Pharm Bull. 2001;24:488-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Dai Y, Liu JX, Li JX, Xu YF. Effect of pinaverium bromide on stress-induced colonic smooth muscle contractility disorder in rats. World J Gastroenterol. 2003;9:557-561. [PubMed] |
| 9. | Curtis AL, Pavcovich LA, Valentino RJ. Long-term regulation of locus ceruleus sensitivity to corticotropin-releasing factor by swim stress. J Pharmacol Exp Ther. 1999;289:1211-1219. [PubMed] |
| 10. | Mönnikes H, Tebbe JJ, Hildebrandt M, Arck P, Osmanoglou E, Rose M, Klapp B, Wiedenmann B, Heymann-Mönnikes I. Role of stress in functional gastrointestinal disorders. Evidence for stress-induced alterations in gastrointestinal motility and sensitivity. Dig Dis. 2001;19:201-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 189] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Gaigé S, Abysique A, Bouvier M. Effects of leptin on cat intestinal motility. J Physiol. 2003;546:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Feinle C, O'Donovan D, Doran S, Andrews JM, Wishart J, Chapman I, Horowitz M. Effects of fat digestion on appetite, APD motility, and gut hormones in response to duodenal fat infusion in humans. Am J Physiol Gastrointest Liver Physiol. 2003;284:G798-G807. [PubMed] |
| 13. | Chang FY, Doong ML, Chen TS, Lee SD, Wang PS. Vasoactive intestinal polypeptide appears to be one of the mediators in misoprostol-enhanced small intestinal transit in rats. J Gastroenterol Hepatol. 2000;15:1120-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Wang QS, Zhang JH. Effects of predator stress on emotional behavior and spatial learning and memory of rats. Chin J psychiatry. 2001;34:180-183. |
| 15. | Ditto B, Miller SB, Barr RG. A one-hour active coping stressor reduces small bowel transit time in healthy young adults. Psychosom Med. 1998;60:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Tsukada F, Sawamura K, Kohno H, Ohkubo Y. Mechanism of inhibition of small intestinal motility by restraint stress differs from that with norepinephrine treatment in rats. Biol Pharm Bull. 2002;25:122-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Serra J, Azpiroz F, Malagelada JR. Impaired transit and tolerance of intestinal gas in the irritable bowel syndrome. Gut. 2001;48:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 243] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Böhme GA, Blanchard JC. Cholecystokinins and their receptors. Functional aspects. Therapie. 1992;47:541-548. [PubMed] |
| 19. | Patterson LM, Zheng H, Berthoud HR. Vagal afferents innervating the gastrointestinal tract and CCKA-receptor immunoreactivity. Anat Rec. 2002;266:10-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Sayegh AI, Ritter RC. CCK-A receptor activation induces fos expression in myenteric neurons of rat small intestine. Regul Pept. 2000;88:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Reidelberger RD, Kelsey L, Heimann D, Hulce M. Effects of peripheral CCK receptor blockade on gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R66-R75. [PubMed] |
| 22. | Hidalgo L, Clavé P, Estorch M, Rodríguez-Espinosa J, Rovati L, Greeley GH, Capellà G, Lluís F. Effect of cholecystokinin-A receptor blockade on postprandial insulinaemia and gastric emptying in humans. Neurogastroenterol Motil. 2002;14:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Lin HC, Zaidel O, Hum S. Intestinal transit of fat depends on accelerating effect of cholecystokinin and slowing effect of an opioid pathway. Dig Dis Sci. 2002;47:2217-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Chey WY, Jin HO, Lee MH, Sun SW, Lee KY. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol. 2001;96:1499-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 244] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 25. | Wu CL, Hung CR, Chang FY, Pau KY, Wang PS. Involvement of cholecystokinin receptor in the inhibition of gastrointestinal motility by estradiol in ovariectomized rats. Scand J Gastroenterol. 2002;37:1133-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Giralt M, Vergara P. Both afferent and efferent nerves are implicated in cholecytokinin motor actions in the small intestine of the rat. Regul Pept. 1999;81:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Patterson LM, Zheng H, Ward SM, Berthoud HR. Immunohistochemical identification of cholecystokinin A receptors on interstitial cells of Cajal, smooth muscle, and enteric neurons in rat pylorus. Cell Tissue Res. 2001;305:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Mao YK, Barnett W, Coy DH, Tougas G, Daniel EE. Distribution of vasoactive intestinal polypeptide (VIP) binding in circular muscle and characterization of VIP binding in canine small intestinal mucosa. J Pharmacol Exp Ther. 1991;258:986-991. [PubMed] |
| 29. | King SC, Slater P, Turnberg LA. Autoradiographic localization of binding sites for galanin and VIP in small intestine. Peptides. 1989;10:313-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Mao YK, Tougas G, Barnett W, Daniel EE. VIP receptors on canine submucosal synaptosomes. Peptides. 1993;14:1149-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Karacay B, O'Dorisio MS, Kasow K, Hollenback C, Krahe R. Expression and fine mapping of murine vasoactive intestinal peptide receptor 1. J Mol Neurosci. 2001;17:311-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Chaudhary P, Baumann TK. Expression of VPAC2 receptor and PAC1 receptor splice variants in the trigeminal ganglion of the adult rat. Brain Res Mol Brain Res. 2002;104:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | You S, Hsu CC, Kim H, Kho Y, Choi YJ, El Halawani ME, Farris J, Foster DN. Molecular cloning and expression analysis of the turkey vasoactive intestinal peptide receptor. Gen Comp Endocrinol. 2001;124:53-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Sayadi H, Harmon JW, Moody TW, Korman LY. Autoradiographic distribution of vasoactive intestinal polypeptide receptors in rabbit and rat small intestine. Peptides. 1988;9:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Usdin TB, Bonner TI, Mezey E. Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology. 1994;135:2662-2680. [PubMed] |
| 36. | Yamamoto H, Kuwahara A, Fujimura M, Maeda T, Fujimiya M. Motor activity of vascularly perfused rat duodenum. 2. Effects of VIP, PACAP27 and PACAP38. Neurogastroenterol Motil. 1999;11:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Hellström PM, Ljung T. Nitrergic inhibition of migrating myoelectric complex in the rat is mediated by vasoactive intestinal peptide. Neurogastroenterol Motil. 1996;8:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Ljung T, Hellström PM. Vasoactive intestinal peptide suppresses migrating myoelectric complex of rat small intestine independent of nitric oxide. Acta Physiol Scand. 1999;165:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Espat NJ, Cheng G, Kelley MC, Vogel SB, Sninsky CA, Hocking MP. Vasoactive intestinal peptide and substance P receptor antagonists improve postoperative ileus. J Surg Res. 1995;58:719-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Hens J, Gajda M, Scheuermann DW, Adriaensen D, Timmermans JP. The longitudinal smooth muscle layer of the pig small intestine is innervated by both myenteric and submucous neurons. Histochem Cell Biol. 2002;117:481-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Näslund E, Backman L, Theodorsson E, Hellström PM. Intraduodenal neuropeptide levels, but not plasma levels, vary in a cyclic fashion with the migrating motor complex. Acta Physiol Scand. 1998;164:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |