Published online Feb 7, 2005. doi: 10.3748/wjg.v11.i5.726
Revised: June 2, 2004
Accepted: June 28, 2004
Published online: February 7, 2005
AIM: To explore the expression effect of mutated IκBα transfection on multidrug resistance gene (MDR-1) in hilar cholangiocarcinoma cells by inhibiting the activity of nuclear transcription factor-κB (NF-κB).
METHODS: We used the mutated IκBα plasmid to transfect QBC939HCVC+ cells and QBC939 cells, and electrophoretic gel mobility shift assay (EMSA) to detect the binding activity of NF-κB DNA and the effect of the transfecting mutated IκBα plasmid on multidrug resistance gene (MDR-1) in hilar cholangiocarcinoma cells and its expression protein (P-GP).
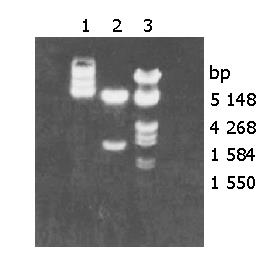
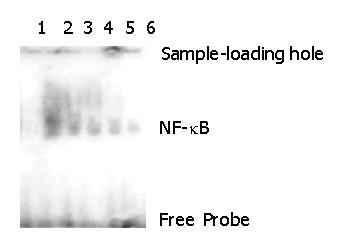
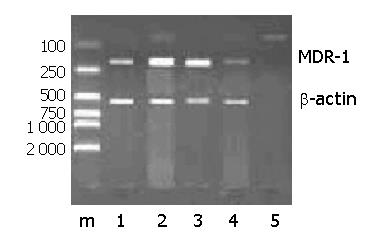
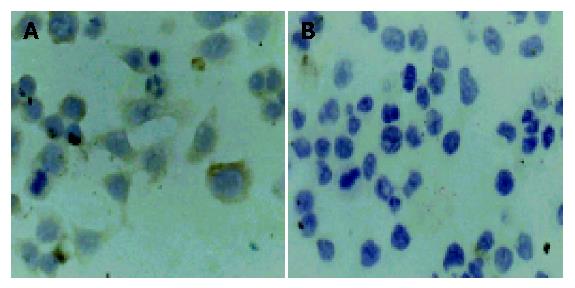
RESULTS: Plasmid DNA was digested by restriction enzymes Xbal and Hand III, and its product after electrophoresis showed two bands with a big difference in molecular weight, with a size of 4.9 kb and 1.55 kb respectively, which indicated that the carrier was successfully constructed and digested with enzymes. The radioactivity accumulation of QBC939HCVC+ and QBC939 cells transfected with mutated IκBα plasmid was significantly lower than that of the control group not transfected with mutated IκBα plasmid. Double densimeter scanning showed that the relative signal density between the tansfection group and non-transfection group was significantly different, which proved that the mutated IκBα plasmid could inhibit the binding activity of NF-κB DNA in hilar cholangiocarcinoma cells. Compared to control group not transfected with m IκBα plasmid, the expression level of MDR-1mRNA in the QBC939 and QBC939HCVC+ cells transfected with mutated IκBα plasmid was lower. The expression intensity of P-GP protein in QBC939 and QBC939HCVC+ cells transfected with mutated IκBα was significantly lower than that of the control group not transfected with mutated IκBα plasmid.
CONCLUSION: The mutated IκBα plasmid transfection can markedly reverse the multidrug resistance of hilar cholangiocarcinoma cells. Interruption of NF-κB activity may become a new target in gene therapy for hilar cholangiocar-cinogenesic carcinoma.
- Citation: Chen RF, Li ZH, Kong XH, Chen JS. Effect of mutated IκBα transfection on multidrug resistance in hilar cholangiocarcinoma cell lines. World J Gastroenterol 2005; 11(5): 726-728
- URL: https://www.wjgnet.com/1007-9327/full/v11/i5/726.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i5.726
With the discovery of the anti-apoptosis effects of nuclear transcription factor-κB (NF-κB) and the research on the activity inhibition of NF-κB, especially the breakthrough progress in the study on the phosphorylation and degradation mechanism of the IκBα of NF-κB, a new way to diagnose and treat hilar cholangiocarcinoma has been explored by inhibiting the activity of NF-κB through gene therapy[1-3]. Mutated IκBα means that the amino acid residues of IκBα at the sites of 32 and 36 are substituted by alanines (S32,36→A), which block the phosphorylation and degradation of IκBα by IKKs, and exclusively bind to the activated NF-κB and subsequently suppress the inappropriate or excessive activation of NF-κB[4-6]. In this paper, we used mutated IκBα to block the activation pathway of NF-κB to explore the effect of mutated IκBα transfection on multidrug resistance (MDR-1) in hilar cholangiocarcinoma cell lines.
QBC939 cells were provided by Professor Wang Shuguang of Southwest Hospital, Third Military Medical University. QBC939HCVC+ cells were prepared by us and stored in our laboratory. E.coli DH5α was stored in our laboratory by general methods. Mutated IκBα (S32, 36→A) plasmid was presented by Professor Baldwin of North Carolina State University. CLONfectin (Clontetch), Trizal (promega), access RT-PCR system (Promega), RNAzol (TEST INC), sense and anti-sense primers of MDR-1 and β2-M, were synthesized by Shanghai Sangon Bioengineering and Technology Service Co., Ltd.
Transformation, identification and maxi preps of mutated IκBαplasmid We used mutated IκBα plasmid to transform the calcified E.coli DH5α, screen the positive clones, amplify and extract and purify the plasmid DNA. The plasmid was stored at -20 °C, for transfection use.
Cell culture and transfection QBC939 cells were incubated in DMEM supplemented with 10% fetal bovine serum (FBS), and mutated IκBα plasmid was transfected to QBC939 and QBC939HCVC +cell lines in log phase using the liposome-mediated method, and compared with the control group.
EMSA test QBC939 and QBC939HCVC +cell lines transfected with mutated IκBα plasmid were examined by electrophoretic gel mobility shift assay (EMSA). Extraction of nuclear protein was referred to the method of Ichikawa et al[7]. The method of Coomassie brilliant blue G250 was used to determine the protein concentration of supernatant. EMSA test was performed according to operation manual of Promega Company provided with the gel shift assay kit.
Detection of expression of MDR-1 gene in transfected cells with RT-PCR The single-step method of guanidine thiocyanate was used to extract the total RNA, which was then quantitated by an UV spectrophotometer and identified by agarose gel electrophoresis. Reverse transcription-PCR reaction was performed in a sensitive single-tube two-enzyme system, (Promega Company). The reaction volume of 50 μL contained 20 μL special RNAase free water, 10 μL AMV/IfI 5, 1 μL dNTP mixture (2.5 mmol/L), 10 μL sense/anti-sense primer mixture, 2 μL MgSO4 (25 mmol/L), 1μL AMV reverse transcriptase (5 u/μL), 1 mL Tfi DNA polymerase (5u/μL), 3 μg RNA sample, MDR-1 sense primer (5’-GTACCCATCATTGCAQATAGC), anti-sense primer (5’- CAAACTTCTGCTCCTGAGTC -3’). PCR reaction conditions were at 94 °C for 40 s, at 56 °C for 45 s; at 72 °C for 1min for 30 cycles. PCR products were identified by 2% agarose gel electrophoresis, and stained with ethidium bromide (EB). Electrophoresis bands of DNA were analyzed with a gel scanner for determining the photon intensity. The ratio of MDR-1 to β 2-M was regarded as the reference data of expression levels to determine the relative quantity of MDR-1 to PCR products.
Immunohistochemistry determination of the expression of P-GP protein in transfection cells The general immunohis-tochemistry adopted labeled-streptavidin biotin system (LSAB), and the positive and negative control groups were set up. When over 5% cytoplasm or cell membrane showed brown-yellow in color, the staining was regarded as the positive P-GP.
Plasmid DNA was digested by restriction enzymes Xbal and HandIII, and its product after electrophoresis showed two bands with a big difference in molecular weight (Figure 1), with a size of 4.9 kb and 1.55 kb respectively, which indicated the carrier was successfully constructed and digested with enzymes.
The radioactivity accumulation of QBC939HCVC+ and QBC939 cells transfected with mutated IκBα plasmid was significantly lower than that of the control group not transfected with mutated IκBα plasmid. Double densimeter scanning showed that the relative signal density between the tansfection group and non-transfection group was significantly different, which proved that the mutated IκBα plasmid had the function of inhibiting the binding activity of NF-κB DNA in hilar cholangiocarcinoma cells.
In order to examine the specificity of EMSA technology, this experiment set up a negative control and a competitive reaction control. The reaction solution of the negative control without addition of the labeled probe showed no band after electrophoresis (Band 3 in Figure 2). The reaction solution of the competitive reaction control after adding the 30× non-labeled probe showed no avidity band of NF-κB after electrophoresis (Band 1 in Figure 1). The experiment indicated that the non-labeled probes bound to NF-κB competitively, causing the labeled probes to decrease remarkably or almost completely. These two experiments proved that the activity detection of NF-κB in this research had a specificity.
Compared with control group, the expression level of MDR-1mRNA in QBC939 and QBC939HCVC+ cells transfected with mutated IκBα plasmid was lower (Figure 3). The expression intensity of P-GP protein in QBC939 and QBC939HCVC+ cells transfected with mutated IκBα was significantly lower than that in the control group not transfected with mutated IκBα plasmid (Figures 4A, B).
IκBα with a molecular weight of 37KD can be divided into three parts. N-terminal domain can be phosphorylated under signal stimulation and is the basic construction to regulate and control the activity of NF-κB. In the core of N-terminal domain, there is a repeat sequence comprised of 5 anchored proteins, which are the binding sites of NF-κB. The C-terminal domain is composed of Pro-Glu-Ser-Thr sequence, which involves in the basic conversion of proteins[8,9].
In 1995 and 1996, the studies on the mechanism of IκBα phosphorylation and degeneration made a breakthrough progress, showing that Ser32 and Ser36 play a vital role in the IκBα phosphorylation and the activation of NF-κB. Ser32 and Ser36 after mutation are not phosphorylated and degenerated, so that they combine firmly with NF-κB to stop NF-κB from entering into the cell nuclei for nuclear transcription[10]. Therefore, some researchers constructed IκBα with site mutations at Ser32 and Ser36, which could be used as the super inhibitors. Compared with the IκBα of wild type, the Ser32 and Ser36 the IκBα of mutated type are replaced by alanines, the mutated IκBα is out of the control of IκBα, continually producing mutated IκBα, and combines with NF-κB to form a trimer, reducing the activation level of NF-κB, which provides a perfect tool for studying the regulation and control of NF-κB.
The expression of multidrug resistance (MDR) gene in tumor cells is the most crucial obstacle for successful chemical therapy. Once cancer cells express the multidrug resistance gene, drugs with different structures used for chemical therapy would produce drug resistance. MDR-1 is a kind of tansmembrane protein P-GP (P-glycoprotein) with a molecular weight of 170kD[11]. P-GP spreads in tissues. It has been reported that 60% tissues of hilar cholangiocarcinoma could express MDR-1. There are NF-κB binding sites in the prompter sequence of MDR-1 gene, MDR-1 is under the regulation and control of NF-κB, which could activate the expression of MDR gene. Therefore, it could be supposed that inhibition of NF-κB activity may increase the accumulation of poisonous drugs between cells to improve the therapeutic effect.
We have proved that the super activation state of NF-κB exists in tissues and cells of hilar cholangiocarcinoma. There is evidence that the function of numerous chemical therapeutic drugs for tumor cells is achieved by promoting apoptosis. However, the drugs for chemical therapy can at the same time activate NF-κB in tumor cells, and activated NF-κB can motivate the expression of various anti-apoptosis genes, and reduce the effect of chemical therapeutic drugs. Consequently, people presume that inhibition of NF-κB can act as an enhancer of chemical therapeutic drugs. Mutated IκBα plasmid, a inhibitor of NF-κB, can markedly reduce the expression of MDR-1. The expression of MDR-1 after transfected with mutated IκBα plasmid is significantly decreased.
Assistant Editor Guo SY Edited by Wang XL
| 1. | Stroh C, Held J, Samraj AK, Schulze-Osthoff K. Specific inhibition of transcription factor NF-kappaB through intracellular protein delivery of I kappaBalpha by the Herpes virus protein VP22. Oncogene. 2003;22:5367-5373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Mi J, Li ZY, Ni S, Steinwaerder D, Lieber A. Induced apoptosis supports spread of adenovirus vectors in tumors. Hum Gene Ther. 2001;12:1343-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Ross JS, Kallakury BV, Sheehan CE, Fisher HA, Kaufman RP, Kaur P, Gray K, Stringer B. Expression of nuclear factor-kappa B and I kappa B alpha proteins in prostatic adenocarcinomas: correlation of nuclear factor-kappa B immunoreactivity with disease recurrence. Clin Cancer Res. 2004;10:2466-2472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Fujioka S, Sclabas GM, Schmidt C, Niu J, Frederick WA, Dong QG, Abbruzzese JL, Evans DB, Baker C, Chiao PJ. Inhibition of constitutive NF-kappa B activity by I kappa B alpha M suppresses tumorigenesis. Oncogene. 2003;22:1365-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Xiong HQ, Abbruzzese JL, Lin E, Wang L, Zheng L, Xie K. NF-kappaB activity blockade impairs the angiogenic potential of human pancreatic cancer cells. Int J Cancer. 2004;108:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Wang JH, Huang QK, Chen MX. Nuclear factor kappa B activity and cell viability of SMMC-7721 inhibited by mutated inhibitor kappa B alpha. Zhonghua GanZangBing ZaZhi. 2003;11:222-224. [PubMed] |
| 7. | Ichikawa K, DeGroot LJ, Refetoff S, Horwitz AL, Pollak ER. Nuclear thyroid hormone receptors in cultured human fibroblasts: improved method of isolation, partial characterization, and interaction with chromatin. Metabolism. 1986;35:861-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Gilmore T, Gapuzan ME, Kalaitzidis D, Starczynowski D. Rel/NF-kappa B/I kappa B signal transduction in the generation and treatment of human cancer. Cancer Lett. 2002;181:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Chen Y, Vallee S, Wu J, Vu D, Sondek J, Ghosh G. Inhibition of NF-kappaB activity by IkappaBbeta in association with kappaB-Ras. Mol Cell Biol. 2004;24:3048-3056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Kabouridis PS, Hasan M, Newson J, Gilroy DW, Lawrence T. Inhibition of NF-kappa B activity by a membrane-transducing mutant of I kappa B alpha. J Immunol. 2002;169:2587-2593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |