Published online Feb 7, 2005. doi: 10.3748/wjg.v11.i5.695
Revised: June 22, 2004
Accepted: July 27, 2004
Published online: February 7, 2005
AIM: To determine the concentrations of leptin in plasma and gastric fundic mucosa in humans, with reference to Helicobacter pylori (H pylori) infection, and their association with gastric mucosal levels of interleukin (IL)-1β, IL-6 and IL-8.
METHODS: Plasma leptin concentrations were determined in 135 outpatients with non-ulcer dyspepsia, consisting of 95 H pylori-infected and 40 uninfected subjects, and 13 patients before and after cure of the infection with anti-H pylori regimen. Using biopsy samples that were endoscopically obtained from the middle corpus along the greater curvature, gastric leptin contents were measured by radioimmunoassay and the mucosal concentrations of IL-1β, IL-6 and IL-8 were measured by enzyme linked immunosorbent assay. We also analysed the expression of leptin in the fundic mucosa by reverse transcriptase-polymerase chain reaction (RT-PCR) and immunohistochemistry.
RESULTS: The mucosal levels of leptin in the fundic mucosa of H pylori-infected patients were significantly higher than those of uninfected patients. The amount of gastric leptin correlated positively with the mucosal levels of IL-1β and IL-6, but not IL-8. Circulating leptin correlated with body mass index, but not with H pylori status, and there was no change in plasma leptin levels following cure of the infection. Leptin immunoreactive cells were noted in the lower half of the fundic glands, and its expression of messenger ribonucleic acid in the oxyntic mucosa was detected by RT-PCR.
CONCLUSION: Leptin production is enhanced in H pylori-infected gastric mucosa. Gastric leptin may be involved in immune and inflammatory response during H pylori infection, through interaction with proinflammatory cytokines.
-
Citation: Nishi Y, Isomoto H, Uotani S, Wen CY, Shikuwa S, Ohnita K, Mizuta Y, Kawaguchi A, Inoue K, Kohno S. Enhanced production of leptin in gastric fundic mucosa with
Helicobacter pylori infection. World J Gastroenterol 2005; 11(5): 695-699 - URL: https://www.wjgnet.com/1007-9327/full/v11/i5/695.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i5.695
Leptin is a 16-kDa protein synthesized mainly by the adipose tissue and plays a crucial role in homeostasis of body weight by reducing appetite and increasing energy expenditure[1,2]. Originally thought to be a satiety factor, leptin is a pleiotropic bioactive molecule[3,4]. Recent evidence demonstrates that leptin regulates immune functions and inhibits gastric acid secretion[3-7]. Contrary to initial reports, leptin production is not restricted to adipocytes. It is also detected in human placenta, muscles and gastric chief cells[3,4,8-10].
Helicobacter pylori (H pylori) is the major cause of chronic gastritis and peptic ulcer diseases[11,12]. Chronic infection leads to atrophic gastritis, which increases the risk of gastric adenocarcinoma[12]. It is well documented that H pylori-associated gastritis, which is characterized by intense infiltration of polynuclear and mononuclear cells, affects various cell types in gastric wall including chief cells[13-15]. Studies from our laboratories and those of other investigators have documented the presence of high concentrations of various proinflammatory cytokines and chemokines in gastric mucosa infected with H pylori[16-20]. Ghrelin, a novel endogenous ligand for growth hormone secretagogue receptor, not only exerts potent growth hormone releasing activity but also influences appetite, energy balance, gastric motility and acid secretion[21]. This hormone is primarily produced by X/A-like neuroendocrine cells in the oxyntic gland[21]. To date, conflicting results have been reported regarding the influence of H pylori status on ghrelin dynamics[22-24]. In terms of the relationship between H pylori infection and leptin, recent studies have shown that gastric leptin contents are higher in H pylori-infected than in uninfected subjects, whereas the serum levels are not always different between the two groups[9,13].
The present study was designed to determine the influence of H pylori status on plasma and gastric levels of leptin. We detected the presence of leptin messenger ribonucleic acid (mRNA) and protein in the gastric mucosa by reverse transcriptase-polymerase chain reaction (RT-PCR) and immunohistochemistry, respectively. In addition, we assessed the relationship between gastric leptin contents and levels of representative proinflammatory cytokines and chemokines including interleukin (IL)-1β, IL-6 and IL-8.
The study subjects were 135 outpatients, who were referred for upper gastrointestinal endoscopy and diagnosed as having non-ulcer dyspepsia, between April 2000 and March 2003. The study was approved by Nagasaki University Human Ethics Committee. All samples were obtained with written informed consent of the patients prior to their inclusion, in accordance with the Helsinki Declaration. The exclusion criteria were: age <18 or >80 years, pregnancy, body mass index (BMI) > 30 kg/m2, diabetes mellitus, systemic infection, thyroid and liver diseases, renal impairment, use of medications effective against H pylori during the preceding 3 mo, alcohol abuse, drug addiction, and long-term corticosteroid or nonsteroidal anti-inflammatory drug use. None had undergone gastrointestinal surgery.
During endoscopy, one biopsy specimen was obtained from the middle portion of the corpus along the greater curvature for the measurement of gastric leptin contents and cytokines, snap-frozen in an ethanol-dry ice mixture and then stored at -80 °C until use. Two additional biopsies were endoscopically taken from the antrum within 2 cm of the pyloric ring and the corpus along the greater curvature; one was for the rapid urease test (Helicocheck, Otsuka Pharmaceutical Co., Tokushima, Japan) and another for histolopathological examination. In some cases, additional biopsy samples were obtained from the fundic gland mucosa for RT-PCR and immunohistochemical analysis.
We treated 13 H pylori-positive patients with 7-d triple therapy consisting of rabeprazole, amoxicillin and clarithromycin[21]. Four weeks after cessation of the treatment, fasting plasma samples were also collected.
On the day of endoscopy, blood samples were taken between 9 and 11 a.m. after an overnight fast, transferred into chilled tubes containing ethylenediaminetetraacetic acid-2Na and aprotinin, stored on ice during collection, centrifuged, plasma separated, and stored at -80 °C until assay. Plasma leptin concentrations were measured in duplicate by a commercial radioimmunoassay (RIA) kit (Linco Research Co., St. Charles, USA), based on the protocol provided by the manufacturer.
As described previously[16,17,26], biopsy samples were homogenized in phosphate-buffered saline (PBS) and aliquots of homogenate supernatants obtained by centrifugation (10000 g for 10 min), were assayed for total protein by a modified Lowry method. The supernatants diluted to 0.50 mg/mL total protein concentration in PBS, were frozen at -80 °C until assay. Gastric leptin contents were measured by RIA (Linco Research). Mucosal levels of IL-1β, IL-6 and IL -8 were measured using commercially available assay kits (Research and Diagnostics Co., Minneapolis, MN, USA), which employ the quantitative immunometric sandwich enzyme immunoassay technique. These assays were performed in duplicate according to the instructions provided by each manufacturer. The concentrations of leptin and cytokines were expressed in ng/mg protein and pg/mg protein, respectively.
H pylori status was assessed by anti-H pylori immunoglobulin G antibody (HEL-p TEST, an enzyme linked immunosorbent assay kit, AMRAD Co., Melbourne, Australia) using the stored plasma, 13C-urea breath test (UBiT, Otsuka Pharmaceutical Co.) and rapid urease test using endoscopic biopsy samples. Patients were considered to be positive for H pylori infection when two of these examinations yielded positive results. On the other hand, patients were defined as H pylori-negative if all test results were negative[27]. Eradication of H pylori was considered successful when 13C-urea breath test became negative[25].
The sections were stained with hematoxylin and eosin. The grades of histological gastritis including activity (neutrophils) and chronic inflammation (mononuclear cells), was scored into 0, 1, 2 or 3 corresponding to none, mild, moderate or severe in accordance with the Sydney system[16].
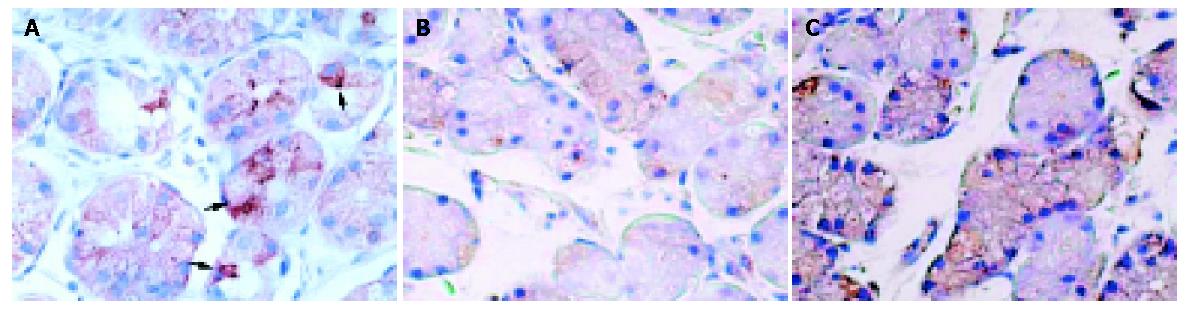
Immunohistochemical staining was performed with the streptavidin-biotin-peroxidase-complex method (Histofine SAB-PO® kit, Nichirei Co., Tokyo, Japan) as described previously[16,17,26]. The following steps were performed at room temperature unless otherwise specified. Paraffin-embedded biopsy specimens were sectioned at 4-μm thickness, deparaffinized and rehydrated. After inhibition of endogenous peroxidase activity for 30 min with methanol containing 0.3% H2O2, the sections were reacted for 20 min with 10% normal goat serum to prevent non-specific binding. They were then incubated overnight with the rabbit polyclonal anti-leptin antibody (diluted 1:100, Santa Cruz Biotechnologies Inc., Santa Cruz, CA, USA) at 4 °C. On the next day, the sections were washed in 0.01 M PBS and incubated for 20 min with 10 mg/mL biotinylated goat anti-rabbit immunoglobulins (Nichirei Co.). After washed in PBS, the sections were re-incubated for 20 min with 100 μg/mL horseradish peroxidase (HRP)-conjugated streptavidin (Nichirei Co.) and stained with 0.02% 3,3’-diaminobenzidine tetrahydrochloride (Dojindo Co., Kumamoto, Japan) in 0.05 mol/L Tris-HCl buffer containing 0.03% H2O2. The sections were finally washed in PBS and counterstained with hematoxylin. Control studies were performed with normal rabbit serum or anti-leptin antiserum (Santa Cruz Biotechnologies Inc.).
We calculated the leptin-labelling index, which was the numbers of immunoreactive cells for leptin per total numbers of cells within the fundic gland area (percentage). The calculation was performed by two investigators without knowledge of the experimental results.
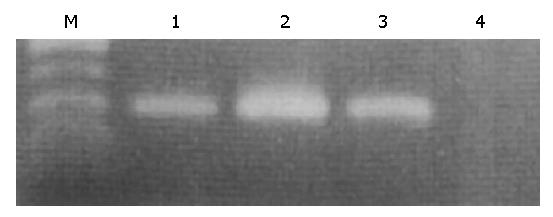
Total RNA from each biopsy sample was extracted using a commercial kit according to the instructions provided by the supplier (ISOGEN, Nippon Gene Co., Toyama, Japan). Equivalent amounts of RNA were monitored by absorption at 260 nm and by monitoring the density of 28S and 18S RNA detected after electrophoresis. One μg of total RNA was reverse transcribed into complementary deoxyribonucleic acid (cDNA) in a volume of 25 μL with MuLV reverse transcriptase and random hexamers (both from PE Applied Biosystems, Warrington, UK). According to a previous report[10] with a slight modification, the target sequence for leptin mRNA was amplified in 40 cycles, each consisting of 1 min at 94 °C for denaturation, 1 min at 62 °C for annealing and 1 min at 72 °C for extension, followed by a final extension for 10 min at 72 °C using a RT-PCR kit (Takara Shuzo Co., Otsu, Japan). Two primers, 5’-CCTGACCTTATCCAAGATGG-3’, (forward) and 5’-GAGTAGCCTGAAGCTTCCAG (reverse), were used for amplification of a 224 bp product[16]. A 10 μL aliquot of each PCR product was analysed by electrophoresis on 2% agarose gel containing ethidium bromide, and the bands were examined under ultraviolet light for the presence of amplified DNA. Glyceraldehyde-3-phosphate dehydrogenase (G3PDH) gene transcript was routinely amplified as described previously[24] and used as an internal control of the processed RNA for each preparation.
Statistical analyses were performed using Fisher’s exact, χ2, Student’s t, Mann-Whitney U, Kruskal-Wallis, Spearman rank and Wilcoxon signed ranks tests, as appropriate. A P value less than 0.05 was accepted as statistically significant. Data were expressed as mean±SD.
The study population consisted of 69 men and 66 women, with a mean age of 54 years (range, 19-80). They consisted of 95 H pylori-infected and 40 uninfected subjects. There were no significant differences in age, sex, alcohol intake, smoking habit, BMI in terms of H pylori status.
There was no significant difference in plasma leptin concentrations between H pylori-positive than -negative subjects (5.0±3.5 and 4.8±2.8 ng/mL, respectively). Successful eradication of the organism was confirmed in all of the 13 patients treated with anti-H pylori regimen. There was no significant difference in the leptin levels before and after cure of the infection (4.8±3.3 and 4.7±2.5 ng/mL, respectively).
Gastric leptin contents in patients with H pylori infection were significantly higher than those in uninfected subjects (P<0.05, Table 1). In addition, there were significant differences in the mucosal levels of IL-6 and IL-8 between H pylori-positive and -negative groups (P<0.0001 and P<0.0005, respectively, Table 1). The mucosal IL-1β concentrations in H pylori-infected patients tended to be higher than those in uninfected subjects, though the difference was insignificant (Table 1). There were no relationships between these cytokines and circulating leptin concentrations.
| H pylori-infected (n = 95) | Uninfected (n = 40) | P | |
| Leptin(ng/mg protein) | 0.18±0.13 | 0.14±0.15 | <0.05 |
| Interleukin 1b (pg/mg protein) | 43.38±33.03 | 33.27±24.61 | NS |
| Interleukin 6 (pg/mg protein) | 1.26±0.57 | 0.75±0.57 | <0.0001 |
| Interleukin 8 (pg/mg protein) | 70.42±66.12 | 1.3±0.13 | <0.0005 |
Gastric leptin contents correlated positively with the mucosal levels of IL-1β (correlation coefficient, r = 0.600, P<0.0001) and IL-6 (r = 0.475, P<0.0005), but not the mucosal levels of IL-8 (r = 0.168).
Gastric leptin contents correlated positively with grading scores of chronic inflammation of gastritis (correlation coefficient, r = 0.258, P<0.05), but not the scores of activity (r = 0.111).
Concentrations of leptin in plasma, but not in gastric mucosa, correlated positively with BMI (r = 0.548, P<0.0001). Other baseline characteristics including age, sex, alcohol intake and smoking habit did not correlate with circulating and gastric leptin concentrations.
Using RT-PCR, leptin mRNA was identified in the fundic gland mucosa, albeit the band intensities of the gastric mucosa from patients with and without H pylori infection were much weaker compared to that of omental fatty tissue (Figure 1).
In gastric biopsy specimens, leptin immunoreactive cells were detected in the lower half of the fundic glands (Figure 2). The leptin-labelling indices of H pylori-infected patients tended to be higher than those of uninfected subjects (22.4±14.3 and 18.1±13.9, respectively, P<0.10).
Bado and co-workers [29] were the first group to report the presence of leptin mRNA and protein in the fundic glands of rat stomachs, and that the chief cells were mainly immunoreactive for leptin[8]. Earlier studies failed to identify leptin mRNA in human gastric mucosa whereas the fundic epithelium exhibited the presence of immunoreactive leptin. Thus, it is suggested that leptin itself detected in gastric mucosa originates from the uptake of circulating one rather than representing local biosynthesis. However, we demonstrated here the expression of both leptin mRNA and its protein, providing further support for the recent results that leptin was a stomach-derived hormone in humans[9,10].
In our study, gastric leptin contents in H pylori-infected patients were significantly higher than those in uninfected patients. Using quantitative RT-PCR, Azuma et al[9] demonstrated that H pylori infection significantly increased the expression of leptin mRNA expression, and that cure of the infection significantly reduced it. Considered together, H pylori infection seems to enhance local biosynthesis of leptin as well as its release into gastric juice in response to cholecystokinin or meal[7]. The finding that leptin was localized in the oxyntic gland area[8-10], which is rarely colonized by the organism[13], suggests that H pylori itself may not affect gastric leptin levels.
Our results demonstrated significantly positive correlations between gastric leptin contents and the mucosal concentrations of IL-1β and IL-6. In fact, there is evidence for the expression of functional leptin receptor (Ob-R) in mononuclear cells[3,4,10,29,30]. Ob-R is homologous to members of class I cytokine receptor (gp130) superfamily including IL-6[3,4,31]. Several lines of evidence demonstrate that leptin could stimulate monocytes to produce IL-1, IL-6 and tumour necrosis factor α (TNF-α)[5,6]. In turn, these cytokines could increase systemic leptin levels in vivo[32]. It has been reported that IL-1β and IL-6 are elevated in gastric mucosa infected with H pylori[18-20], in line with this study. Thus, leptin released locally may be implicated in the immune and inflammatory responses to H pylori infection, through interaction with proinflammatory cytokines. Moreover, it not only modulates the activation and proliferation of T lymphocytes but also skews cytokine responses towards a Th 1 phenotype by enhancing production of IL-2 and interferon γ[33,34]. It has been well accepted that mucosal cytokine profiles during H pylori infection can imply Th1 predominance in human adults[13]. Considered together, these findings highlight the possible role of leptin as an immunomodulator in H pylori-associated gastritis.
In our study, circulating leptin concentrations were not associated with H pylori status and there was no significant alteration in their levels following cure of the infection, consistent with previous reports[19,35]. Gastric leptin may have a local rather than a systemic action, exerting paracrine effects within the gastric mucosa. On the other hand, plasma leptin concentrations significantly correlated with BMI, as the primary contributor of circulating leptin is exclusively the adipose tissue[1,2].
In conclusion, we showed a significantly enhanced production of leptin in H pylori-infected than uninfected gastric mucosa. The amount of gastric leptin correlated positively with the mucosal concentrations of IL-1β and IL-6, suggesting that local overproduction of leptin is likely to be involved in immune and inflammatory response during H pylori infection.
Edited by Wang XL
| 1. | Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9119] [Cited by in RCA: 8855] [Article Influence: 285.6] [Reference Citation Analysis (0)] |
| 2. | Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 900] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 3. | Faggioni R, Feingold KR, Grunfeld C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. FASEB J. 2001;15:2565-2571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 327] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 4. | Sánchez-Margalet V, Martín-Romero C, Santos-Alvarez J, Goberna R, Najib S, Gonzalez-Yanes C. Role of leptin as an immunomodulator of blood mononuclear cells: mechanisms of action. Clin Exp Immunol. 2003;133:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 253] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol. 1999;194:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 427] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 6. | Zarkesh-Esfahani H, Pockley G, Metcalfe RA, Bidlingmaier M, Wu Z, Ajami A, Weetman AP, Strasburger CJ, Ross RJ. High-dose leptin activates human leukocytes via receptor expression on monocytes. J Immunol. 2001;167:4593-4599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 221] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Konturek JW, Konturek SJ, Kwiecień N, Bielański W, Pawlik T, Rembiasz K, Domschke W. Leptin in the control of gastric secretion and gut hormones in humans infected with Helicobacter pylori. Scand J Gastroenterol. 2001;36:1148-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y. The stomach is a source of leptin. Nature. 1998;394:790-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 769] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 9. | Azuma T, Suto H, Ito Y, Ohtani M, Dojo M, Kuriyama M, Kato T. Gastric leptin and Helicobacter pylori infection. Gut. 2001;49:324-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Sobhani I, Bado A, Vissuzaine C, Buyse M, Kermorgant S, Laigneau JP, Attoub S, Lehy T, Henin D, Mignon M. Leptin secretion and leptin receptor in the human stomach. Gut. 2000;47:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 215] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Blaser MJ. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990;161:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 478] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 12. | Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol. 2000;54:615-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 398] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 13. | Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease. J Clin Invest. 2004;113:321-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 621] [Article Influence: 29.6] [Reference Citation Analysis (1)] |
| 14. | Beales IL, Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut. 1998;42:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 211] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Moss SF, Legon S, Bishop AE, Polak JM, Calam J. Effect of Helicobacter pylori on gastric somatostatin in duodenal ulcer disease. Lancet. 1992;340:930-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 178] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Isomoto H, Mizuta Y, Miyazaki M, Takeshima F, Omagari K, Murase K, Nishiyama T, Inoue K, Murata I, Kohno S. Implication of NF-kappaB in Helicobacter pylori-associated gastritis. Am J Gastroenterol. 2000;95:2768-2776. [PubMed] |
| 17. | Isomoto H, Miyazaki M, Mizuta Y, Takeshima F, Murase K, Inoue K, Yamasaki K, Murata I, Koji T, Kohno S. Expression of nuclear factor-kappaB in Helicobacter pylori-infected gastric mucosa detected with southwestern histochemistry. Scand J Gastroenterol. 2000;35:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Crabtree JE, Shallcross TM, Heatley RV, Wyatt JI. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991;32:1473-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 397] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 19. | Noach LA, Bosma NB, Jansen J, Hoek FJ, van Deventer SJ, Tytgat GN. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994;29:425-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 350] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology. 1996;110:1744-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 236] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 21. | Murray CD, Kamm MA, Bloom SR, Emmanuel AV. Ghrelin for the gastroenterologist: history and potential. Gastroenterology. 2003;125:1492-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Gokcel A, Gumurdulu Y, Kayaselcuk F, Serin E, Ozer B, Ozsahin AK, Guvener N. Helicobacter pylori has no effect on plasma ghrelin levels. Eur J Endocrinol. 2003;148:423-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Nwokolo CU, Freshwater DA, O'Hare P, Randeva HS. Plasma ghrelin following cure of Helicobacter pylori. Gut. 2003;52:637-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 164] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Suzuki H, Masaoka T, Hosoda H, Ota T, Minegishi Y, Nomura S, Kangawa K, Ishii H. Helicobacter pylori infection modifies gastric and plasma ghrelin dynamics in Mongolian gerbils. Gut. 2004;53:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Isomoto H, Furusu H, Morikawa T, Mizuta Y, Nishiyama T, Omagari K, Murase K, Inoue K, Murata I, Kohno S. 5-day vs. 7-day triple therapy with rabeprazole, clarithromycin and amoxicillin for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2000;14:1619-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Isomoto H, Wang A, Mizuta Y, Akazawa Y, Ohba K, Omagari K, Miyazaki M, Murase K, Hayashi T, Inoue K. Elevated levels of chemokines in esophageal mucosa of patients with reflux esophagitis. Am J Gastroenterol. 2003;98:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Isomoto H, Inoue K, Furusu H, Nishiyama H, Shikuwa S, Omagari K, Mizuta Y, Murase K, Murata I, Kohno S. Lafutidine, a novel histamine H2-receptor antagonist, vs lansoprazole in combination with amoxicillin and clarithromycin for eradication of Helicobacter pylori. Helicobacter. 2003;8:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Ohara H, Isomoto H, Wen CY, Ejima C, Murata M, Miyazaki M, Takeshima F, Mizuta Y, Murata I, Koji T. Expression of mucosal addressin cell adhesion molecule 1 on vascular endothelium of gastric mucosa in patients with nodular gastritis. World J Gastroenterol. 2003;9:2701-2705. [PubMed] |
| 29. | Breidert M, Miehlke S, Glasow A, Orban Z, Stolte M, Ehninger G, Bayerdörffer E, Nettesheim O, Halm U, Haidan A. Leptin and its receptor in normal human gastric mucosa and in Helicobacter pylori-associated gastritis. Scand J Gastroenterol. 1999;34:954-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Madej T, Boguski MS, Bryant SH. Threading analysis suggests that the obese gene product may be a helical cytokine. FEBS Lett. 1995;373:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 156] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2526] [Cited by in RCA: 2431] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 32. | Sarraf P, Frederich RC, Turner EM, Ma G, Jaskowiak NT, Rivet DJ, Flier JS, Lowell BB, Fraker DL, Alexander HR. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185:171-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 568] [Cited by in RCA: 557] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 33. | Lord GM, Matarese G, Howard JK, Bloom SR, Lechler RI. Leptin inhibits the anti-CD3-driven proliferation of peripheral blood T cells but enhances the production of proinflammatory cytokines. J Leukoc Biol. 2002;72:330-338. [PubMed] |
| 34. | Picker LJ, Singh MK, Zdraveski Z, Treer JR, Waldrop SL, Bergstresser PR, Maino VC. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995;86:1408-1419. [PubMed] |
| 35. | Shimzu T, Satoh Y, Yamashiro Y. Serum leptin and body mass index in children with H pylori infection. Gut. 2002;51:142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |