Published online Feb 7, 2005. doi: 10.3748/wjg.v11.i5.676
Revised: April 27, 2004
Accepted: May 9, 2004
Published online: February 7, 2005
AIM: To determine the possible existence of a hepato-cardiovascular response and its regulatory mechanism in normal rats.
METHODS: Systemic hemodynamic changes following intraportal injection of latex microspheres were studied in two modified rat models of hepatic circulation, in which the extrahepatic splanchnic circulation was excluded by evisceration and the liver was perfused by systemic blood via either the portal vein (model 1) or hepatic artery (model 2) in vivo.
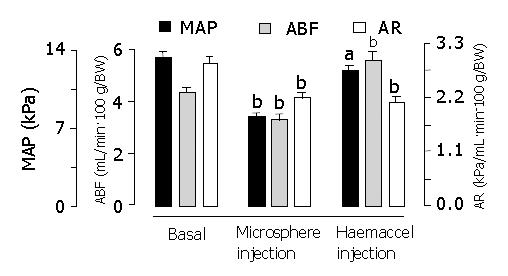
RESULTS: In model 1, intraportal injection of two sized microspheres (15-μm and 80-μm) induced a similar decrease in mean arterial pressure, while extrahepatic portal venous occlusion induced an immediate increase in mean arterial pressure. In model 2, microsphere injection again induced a significant reduction in mean arterial pressure, aortic blood flow and aortic resistance. There were no significant differences in these parameters between liver-innervated rats and liver-denervated rats. The degrees of microsphere-induced reduction in mean arterial pressure (-38.1±1.9% in liver-innervated rats and -35.4±2.1% in liver-denervated rats, respectively) were similar to those obtained by withdrawal of 2.0 mL of blood via the jugular vein (-33.3±2.1%) (P>0.05). Injection of 2.0 mL Haemaccel in microsphere-treated rats, to compensate for the reduced effective circulating blood volume, led to a hyperdynamic state which, as compared with basal values and unlike control rats, was characterised by increased aortic blood flow (+21.6±3.3%), decreased aortic resistance (-38.1±3.5%) and reduced mean arterial pressure (-9.7±2.8%).
CONCLUSION: A hepato-cardiovascular response exists in normal rats. It acts through a humoral mechanism leading to systemic vasodilatation, and may be involved in the hemodynamic disturbances associated with acute and chronic liver diseases.
- Citation: Li XN, Benjamin IS, Alexander B. Hepato-cardiovascular response and its regulation. World J Gastroenterol 2005; 11(5): 676-680
- URL: https://www.wjgnet.com/1007-9327/full/v11/i5/676.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i5.676
Several mechanisms are involved in the regulation of hepatic hemodynamics, which include distensible hepatic resistance[1], changeable hepatic blood volume[2], hepatic arterial buffer response[3] and intrahepatic portal systemic shunts[4-6]. However, a direct hemodynamic relationship between the liver and the cardiovascular system has not been described.
In our previous studies, it was found that intrahepatic portal block by microspheres induced an acute reduction in systemic blood pressure[4-6]. Originally this was attributed to mesenteric pooling of portal venous blood[4-6], but subsequent studies have suggested that this might be due to a hepato-cardiovascular response[7,8]. To further confirm such a response, the present study used eviscerated rats to exclude extrahepatic splanchnic circulation, and the liver was perfused with systemic blood via either the portal vein or hepatic artery in vivo. Thus, if an autoregulatory response mechanism exists between the liver and the systemic circulation, this could be activated directly following appropriate stimulation to the liver. The aims of the present study were to determine whether: (1) intraportal injection of latex microspheres would result in hemodynamic changes in the systemic circulation; (2) different-sized microspheres affected changes in systemic hemodynamics; (3) systemic circulatory responses to the microsphere injection could be induced in the absence of portal flow to the liver; and (4) denervation of the liver altered microsphere-induced responses in systemic hemodynamics.
Male Wistar rats (350-400 g) were anaesthetized with fentanyl/fluanisone (0.3 mL/kg subcutaneously) and midazolam (0.3 mL/kg subcutaneously). The left carotid artery was cannulated for measurement of mean arterial pressure (MAP). Another cannula was inserted into the right jugular vein and advanced 2-3 cm to reach the superior vena cava for measurement of central venous pressure (CVP).
The abdomen was then opened via a midline incision. The gastric artery, splenic artery and superior mesenteric artery were exposed and ligated, and the stomach, intestines and spleen were subsequently removed.
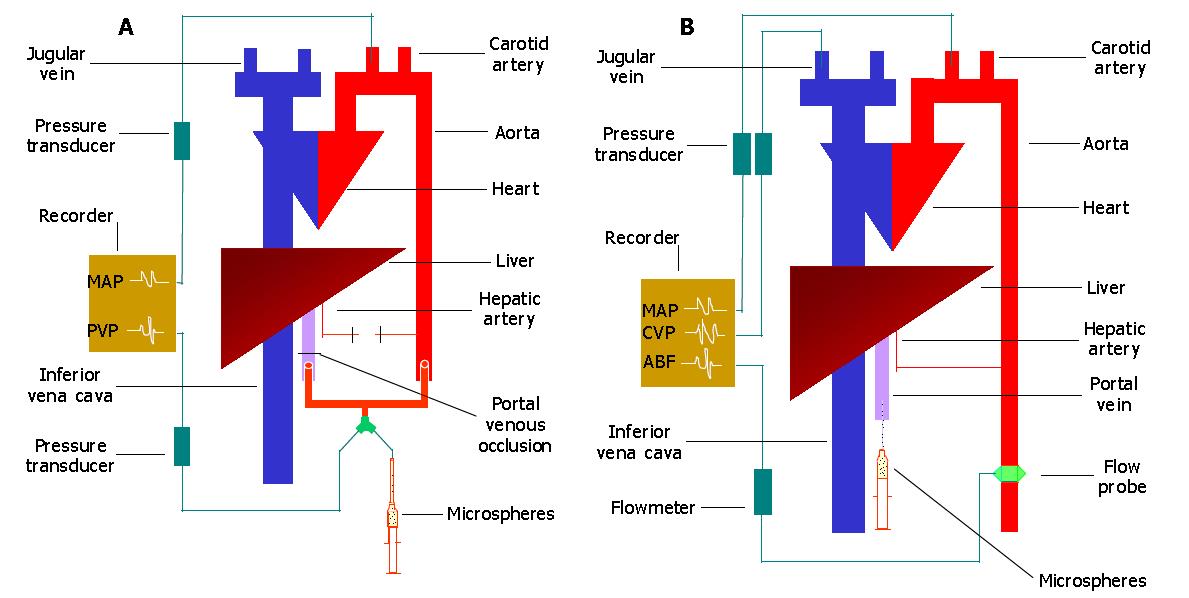
After evisceration, the liver was perfused either by aortic blood via the portal vein in vivo (model 1) or by hepatic arterial blood in vivo (model 2). In model 1, the hepatic artery was carefully dissected and ligated. The main portal vein was divided so that a complete evisceration could be achieved. To re-construct the portal venous supply to the liver, the abdominal aorta was ligated and divided just above its bifurcation and the cephalad end of the aorta was connected to the hepatic end of the portal vein by a T-type connector. The third arm of the connector was used for measurement of portal venous pressure (PVP) and injection of microspheres (Figure 1A). In model 2, the liver was supplied only by the hepatic artery. The portal vein was also divided, and the hepatic remnant cannulated for injection of microspheres (Figure 1B).
Experiment 1 After a 20-min stabilization period, a vascular clamp was applied to the portal vein in model 1 (Figure 1A) for 2-3 min and the pressures recorded as a measurement of the maximum attainable portal venous pressure[4,5]. Five minutes after releasing the vascular clamp, rats were given intraportally via four equal injections (0.2 mL/injection) with a total of 2.2 × 107 15 μm diameter microspheres (group 1, n = 6), or 3.6 × 105 80 μm diameter microspheres (group 2, n = 6)[4,5]. Identical volumes of saline were given intraportally to controls (group 3, n = 6) during the same time course. When steady-state portal venous pressure was obtained, portal venous occlusion (PVO) was performed again. The resultant changes in MAP and PVP were continuously monitored. Because the liver was perfused by aortic blood with high pressure, which might have enlarged the hepatic sinusoids and/or opened intrahepatic portal-systemic shunts[4-6] to lead to pulmonary embolization with a large number of microspheres, an additional group of rats (n = 6) was, therefore, used for measurement of intrahepatic shunting by using 15-μm diameter 51Cr labeled microspheres[6,9].
Experiment 2 In model 2 (Figure 1B), 5.6×106 15-μm diameter microspheres or equivalent volumes of saline (0.2 mL) were injected intraportally, and the resultant changes in MAP, CVP and aortic blood flow (ABF) were measured in liver-innervated rats (group 1, n = 8), liver-denervated rats[10] (group 2, n = 8) and control rats (group 3, n = 8) respectively. ABF was measured by using an electromagnetic flow transducer that was placed around the abdominal aorta between the celiac axis and renal arteries. Results obtained were compared with that of group 4 (n = 6) which underwent sequential blood withdrawals and subsequent Haemaccel injections via the jugular vein (0.5 mL/withdrawal or injection, both up to 6 times). The microsphere-induced hypovolemia, secondary to vasodilatation (see below), was then identified, and an appropriate volume of Haemaccel was given via the jugular vein to rats pre-treated with 15-μm diameter microspheres (5.6×106) injected intraportally (group 5, n = 8).
Aortic blood flow was measured as mL/(min·100 g) body weight. Aortic resistance (AR) was calculated as kPa/aortic blood flow. Intrahepatic shunting was calculated from injection of 15-μm diameter 51Cr labeled microspheres as follows[9]: Intrahepatic shunting (%) = lung radioactivity (cpm)×100/ liver radioactivity (cpm) + lung radioactivity (cpm). The results were expressed as mean ± SE. Statistical comparisons were performed using t test and factorial ANOVA where appropriate unless otherwise stated. Results were considered statistically significant when a P value was <0.05.
Basal MAP and PVP were significantly increased by PVO to a similar level, which returned to basal values immediately after release of PVO (Table 1, Figure 2). In contrast, although microsphere injections in groups 1 and 2 gradually increased PVP due to increased intrahepatic portal resistance, MAP was significantly decreased following the first injection of microspheres in both groups, and showed no further significant changes following the subsequent 3 injections. There were no significant differences in MAP reduction between group 1 and group 2. PVO, following microsphere injections, induced a significantly smaller increase in MAP and PVP than PVO prior to injection of microspheres (Table 1, Figure 2). There were no significant changes in control rats given saline.
Although the liver was perfused via the portal vein by aortic blood with high pressure, intrahepatic shunting was negligible with a mean value of (0.06±0.04)% (0.03-0.10%).
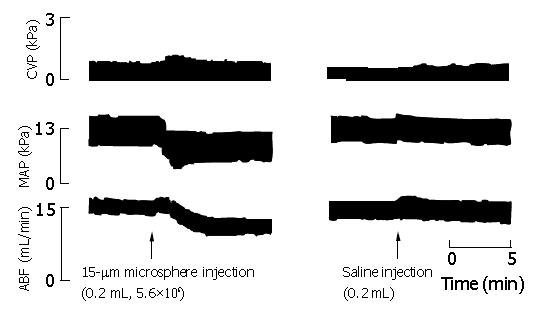
Intraportal injection of microspheres resulted in significant reductions in MAP, ABF and AR, 60-90 s after microsphere injection (Table 2, Figure 3). CVP showed no significant changes following microsphere injection. There were no significant differences in these parameters between liver-innervated rats (group 1) and liver-denervated rats (group 2). No significant changes were observed in control group given saline.
| Pre-injection | Post-injection | Change (%) | |
| CVP (kPa) | |||
| Group 1 | 0.43±0.07 | 0.39±0.06 | -6.9±0.72 |
| Group 2 | 0.40±0.08 | 0.38±0.59 | -5.5±0.66 |
| Group 3 | 0.34±0.06 | 0.35±0.08 | 1.3±0.22 |
| MAP (kPa) | |||
| Group 1 | 12.7±0.4 | 7.9±0.4a | -38.1±1.9 |
| Group 2 | 12.9±0.6 | 8.4±0.5a | -35.4±2.1 |
| Group 3 | 12.9±0.4 | 13.1±0.4 | 1.5±0.2 |
| ABF (mL/min/ 100 g BW) | |||
| Group 1 | 4.2±0.3 | 3.4±0.3a | -21.3±2.6 |
| Group 2 | 3.7±0.2 | 3.1±0.2a | -16.8±2.5 |
| Group 3 | 4.1±0.3 | 4.2±0.3 | 2.1±0.1 |
| AR (kPa/mL/ min/100 g BW) | |||
| Group 1 | 3.1±0.1 | 2.4±0.1a | -21.0±2.3 |
| Group 2 | 3.5±0.1 | 2.7±0.1a | -21.7±2.3 |
| Group 3 | 3.3±0.2 | 3.2±0.2 | -0.4±0.1 |
Sequential blood withdrawal in group 4 caused a parallel decrease in MAP and ABF, which, after subsequent Haemaccel injections, completely returned to basal values. Microsphere-induced MAP reductions in liver-innervated rats (7.9±0.4 kPa) and liver-denervated rats (8.4±0.5 kPa) were comparable to those obtained by withdrawal of 2.0 mL blood via the jugular vein in group 4 (8.3±0.5 kPa) (P>0.05). Therefore 2.0 mL of Haemaccel was infused in microsphere-treated rats in group 5, to compensate for the reduced effective circulating blood volume. This led to a hyperdynamic state which, as compared with the basal values and unlike rats in group 4, was characterized by increased ABF, decreased AR and reduced MAP (Table 3, Figures 4 and 5).
The present study established two modified rat models of hepatic circulation, in which the extrahepatic splanchnic circulation was excluded by evisceration and the liver was perfused by systemic blood via either the portal vein (model 1) or hepatic artery (model 2) in vivo. Therefore, the possible hemodynamic relationship between the liver and the cardiovascular system could be easily observed. Results showed that intraportal injection of microspheres induced a significant reduction in MAP in both models, thus supporting our previous hypothesis that there exists a hepato-cardiovascular response in normal rats[7,8].
In model 1, the liver was only supplied by aortic blood via the portal vein (Figure 1A). It was expected that in this model, either intraportal injection of microspheres or PVO should increase the resistance to aortic blood that was flowing through the portal vasculature, thus leading to elevations in both MAP and PVP. As expected, PVO prior to injection of microspheres, induced an immediate increase in mean arterial pressure. However, injection of microspheres into the liver induced an immediate decrease in MAP. Furthermore, PVO following microsphere injections induced a significantly smaller increase in MAP.
The reduction in MAP might be a result of a major shunting of microspheres into the pulmonary circulation, due to enlarged sinusoids and opened intrahepatic portal-systemic shunts[4,5] caused by a high portal venous perfusion pressure. However, we showed that the percentage of intrahepatic shunting, measured with 15μm diameter labeled microspheres, was negligible (<0.1%). This is consistent with our recent findings that intrahepatic shunts in the normal rat liver are predominantly small with diameters less than 15-μm[6].
The liver is characterized by a high degree of compliance. Therefore the microsphere-induced reductions in MAP could also result from intrahepatic blood pooling, secondary to portal block by the injected microspheres. If so, MAP should decrease progressively with increasing numbers and sizes (diameters) of microspheres as more sinusoids would become occluded in the liver, but this was not observed. In fact, a similar and sustained reduction in MAP was observed in groups 1 and 2 of experiment 1, following the first injection of 15-μm and 80-μm microspheres, respectively. Moreover, significant intrahepatic portal pooling could not occur because the opening of intrahepatic portal systemic shunts (<15-μm) can permit 70% of the basal portal venous blood to bypass the hepatic sinusoids[6]. We, therefore, hypothesize that the reduction in MAP is possibly due to an autoregulatory response elicited by the effects of injected microspheres in the liver.
This hypothesis is supported by the results from the subsequent experiments with model 2, in which the absence of portal flow excluded portal venous pooling as the cause. Again, significant reductions in MAP, aortic blood flow and aortic resistance were induced by intraportal injection of microspheres. These changes strongly suggest a significant systemic vasodilatation leading to reductions in effective circulating blood volume. Furthermore, the microsphere-induced hemodynamic responses are different from those seen in acute toxic liver injury, in which vasodilatation develops over hours or days[11], not in seconds as observed in the present study. It is, therefore, suggested that a hepato-cardiovascular response exists in normal rats, by which the systemic circulation may quickly respond to intrahepatic portal block by injected microspheres.
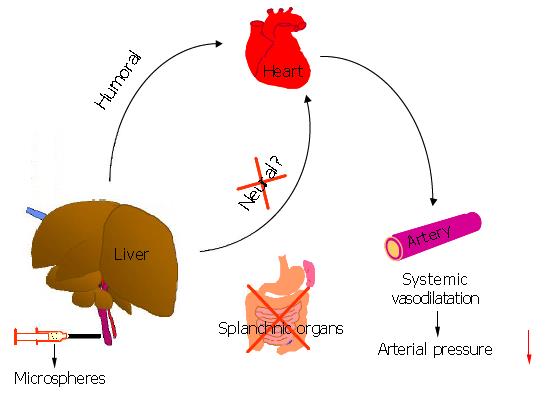
The fact that denervation of the liver does not eliminate this response suggests that a humoral mechanism is involved in the hepato-cardiovascular response, and that, following microsphere injection, the liver releases substance (s) which may exert a potent vasodilator action on the systemic circulation. Although we did not perform humoral measurements as this is beyond the scope of the present study, it is well known that the liver can produce many vasodilators such as nitric oxide[12]. The systemic responses in model 1, in which a large quantity of aortic blood flows through the liver via the portal vein with low resistance, occurred immediately, while in model 2 there was a delay of 60-90 s. This is consistent with rapid flushing of humoral factors into the systemic circulation in model 1.
Microsphere-induced acute systemic vasodilatation resulted in a state of relative hypovolemia and thus a reduction in MAP comparable to withdrawal of 2.0 mL of blood via the jugular vein. Infusion of 2.0 mL Haemaccel, to compensate for the reduced effective circulating blood volume in microsphere-treated rats, led to a hyperdynamic circulation, characterized by increased aortic blood flow, decreased aortic resistance and reduced MAP. Such circulatory disturbances are similar to those observed following acute[11] or chronic liver injuries[13], and consistent with the hypothesis that the combination of vasodilatation and expanded plasma volume is necessary for full expression of the hyperdynamic state[14]. However, the initiating factor for the hyperdynamic circulation appears to be the development of vasodilatation, which evokes an increase in blood volume and cardiac output as a compensatory response[15]. Although the mechanisms underlying the vasodilatation are still not fully understood, we have shown here, for the first time, a direct hemodynamic response between the liver and the cardiovascular system. It is suggested that this response may play a role in the development of vasodilatation observed in acute and chronic liver diseases such as severe hepatitis and cirrhosis, where the mechanical stimulation to the liver, similar to that induced by microsphere injections, is a common feature which results from leukocyte accumulation[16], endothelial oedema[17], Kupffer cell swelling[16], and portal occlusion or compression by scar tissue and regenerative hyperplastic nodules[18,19].
In conclusion, this study demonstrates the existence of a hepato-cardiovascular response in normal rats. This response acts through a humoral mechanism and leads to systemic vasodilatation, and may be involved in the hemodynamic disturbances associated with acute and chronic liver diseases.
Edited by Xia HHX Proofread by Zhu LH
| 1. | Lautt WW, Legare DJ. Passive autoregulation of portal venous pressure: distensible hepatic resistance. Am J Physiol. 1992;263:G702-G708. [PubMed] |
| 2. | Lautt WW, Brown LC, Durham JS. Active and passive control of hepatic blood volume responses to hemorrhage at normal and raised hepatic venous pressure in cats. Can J Physiol Pharmacol. 1980;58:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Lautt WW. Mechanism and role of intrinsic regulation of hepatic arterial blood flow: hepatic arterial buffer response. Am J Physiol. 1985;249:G549-G556. [PubMed] |
| 4. | Li XN, Benjamin I, Alexander B. A new rat model of portal hypertension induced by intraportal injection of microspheres. World J Gastroenterol. 1998;4:66-69. [PubMed] |
| 5. | Li X, Benjamin IS, Alexander B. The relationship between intrahepatic portal systemic shunts and microsphere induced portal hypertension in the rat liver. Gut. 1998;42:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Li X, Benjamin IS, Naftalin R, Alexander B. Location and function of intrahepatic shunts in anaesthetised rats. Gut. 2003;52:1339-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Li X, Benjamin IS, Alexander B. Evidence for a hepato-cardiovascular reflex in the rat. Hepatogastroenterology. 1999;46:1419. |
| 8. | Li X, Benjamin IS, Alexander B. Further evidence for a hepato-cardiovascular reflex: demonstrated in a rat model of hepatic circulation in the absence of portal flow in vivo. Hepatogastroenterology. 1999;46:1448. |
| 9. | Groszmann RJ, Vorobioff J, Riley E. Splanchnic hemodynamics in portal-hypertensive rats: measurement with gamma-labeled microspheres. Am J Physiol. 1982;242:G156-G160. [PubMed] |
| 10. | Holmin T, Ekelund M, Kullendorff CM, Lindfeldt J. A microsurgical method for denervation of the liver in the rat. Eur Surg Res. 1984;16:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Makin AJ, Hughes RD, Williams R. Systemic and hepatic hemodynamic changes in acute liver injury. Am J Physiol. 1997;272:G617-G625. [PubMed] |
| 12. | Li X, Benjamin IS, Alexander B. The role of nitric oxide in systemic and hepatic haemodynamics in the rat in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Bosch J, Enriquez R, Groszmann RJ, Storer EH. Chronic bile duct ligation in the dog: hemodynamic characterization of a portal hypertensive model. Hepatology. 1983;3:1002-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Colombato LA, Albillos A, Groszmann RJ. Temporal relationship of peripheral vasodilatation, plasma volume expansion and the hyperdynamic circulatory state in portal-hypertensive rats. Hepatology. 1992;15:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 98] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1131] [Cited by in RCA: 1022] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 16. | Jaeschke H, Smith CW. Cell adhesion and migration. III. Leukocyte adhesion and transmigration in the liver vasculature. Am J Physiol. 1997;273:G1169-G1173. [PubMed] |
| 17. | Vollmar B, Glasz J, Leiderer R, Post S, Menger MD. Hepatic microcirculatory perfusion failure is a determinant of liver dysfunction in warm ischemia-reperfusion. Am J Pathol. 1994;145:1421-1431. [PubMed] |
| 18. | Li X, Benjamin IS, Alexander B. Reproducible production of thioacetamide-induced macronodular cirrhosis in the rat with no mortality. J Hepatol. 2002;36:488-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Li XN, Huang CT, Wang XH, Leng XS, Du RY, Chen YF, Hou X. Changes of blood humoral substances in experimental cirrhosis and their effects on portal hemodynamics. Chin Med J (Engl). 1990;103:970-977. [PubMed] |