Published online Feb 7, 2005. doi: 10.3748/wjg.v11.i5.645
Revised: May 4, 2004
Accepted: June 29, 2004
Published online: February 7, 2005
AIM: To evaluate the serum levels of cytokeratins and carcinoembryonic antigen (CEA) in diagnosis, staging and prognosis of patients with colorectal adenocarcinoma.
METHODS: The sample consisted of 169 patients. One hundred blood donors formed the control group. Radical surgery was performed on 120 patients, with an average follow-up duration of 22.3 mo. Relapses occurred in 23 individuals after an average of 18.09 mo. CEA was assayed via the Delfia® method with a limit of 5 ng/mL. Cytokeratins were assayed via the LIA-mat® TPA-M Prolifigen® method with a limit of 72 U/L.
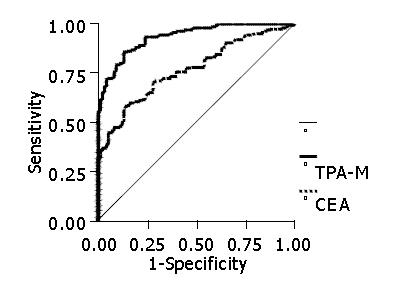
RESULTS: In the diagnosis of patients with colorectal adenocarcinoma, CEA showed a sensitivity of 56%, a specificity of 95%, a positive predictive value of 94%, a negative predictive value of 50% and an accuracy of 76.8%. TPA-M had a sensitivity of 70%, a specificity of 96%, a positive predictive value of 97%, a negative predictive value of 66% and an accuracy of 93.6%. The elevation of one of the markers was shown to have a sensitivity of 76.9%, a specificity of 91%, a positive predictive value of 93.5%, a negative predictive value of 70% and an accuracy of 83.6%. There was no variation in the levels of the markers according to the degree of cell differentiation while there was an elevation in their concentrations in accordance with the increase in neoplastic dissemination. There was a statistically significant difference between the patients with stage IV lesions and those with stages I, II and III tumors. With regard to CEA, the average level was 14.2 ng/mL in patients with stage I lesions, 8.5 ng/mL in patients with stage II lesions, 8.0 ng/mL in patients with stage III lesions and 87.7 ng/mL in patients with stage IV lesions. In relation to TPA-M, the levels were 153.1 U/L in patients with stage I tumors, 106.5 U/L in patients with stage II tumors, 136.3 U/L in patients with stage III tumors and 464.3 U/L in patients with stage IV tumors. There was a statistical difference in patients with a high CEA level in relation to a shorter survival (P<0.05). However, there was no correlation between patients with high TPA-M levels and prognostic indices of patients undergoing radical surgery.
CONCLUSION: Cytokeratins demonstrate a greater sensitivity than CEA in the diagnosis of colorectal adenocarcinoma. There is an increase in the sensitivity of the markers with tumor dissemination. Cytokeratins cannot identify the worse prognosis in patients undergoing radical surgery. Cytokeratins constitute an advance in the direction of a perfect tumor marker in the treatment of patients with colorectal cancer.
- Citation: Fernandes LC, Kim SB, Matos D. Cytokeratins and carcinoembryonic antigen in diagnosis, staging and prognosis of colorectal adenocarcinoma. World J Gastroenterol 2005; 11(5): 645-648
- URL: https://www.wjgnet.com/1007-9327/full/v11/i5/645.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i5.645
Colorectal cancer is the third most frequent cancer in the world, with a high incidence rate in North America, Western Europe, Australia, New Zealand and France[1,2]. The general survival rate of colorectal patients does not exceed 40%[3,4].
The average five-year survival rate of patients with lesions diagnosed at early stages (stage I) is approximately 70%, and is 6% in cases of advanced disease (stage IV)[5]. Better public awareness has assisted in diagnosing lesions at initial stages. Nonetheless, patients are commonly found to have the disease at advanced stages with extremely poor results. Sometimes palliative surgery or interventions are performed in which tumor resection is not achieved[6,7].
It is in this context that the use of serum tumor markers has its place. These substances, which can be detected in peripheral blood indicate the existence of developing neoplasm in the body[8]. In colorectal adenocarcinoma, CEA[9] has become distinguished as a tumor marker in the diagnosis[10-12], staging[10-12], and prognosis[13-15] of patients with colorectal carcinoma, and in the detection of its recurrence[16-19].
Other markers have been developed such as CA 19-9[20,21], CA 242[21], CA 72-4[22], cytokeratins[23,24], VEGF[25], and p53[26,27]. Of these, cytokeratins merit attention.
Tissue polypeptide antigen (TPA) was the first developed for detecting cytokeratins in 1978[23]. This evolved into tissue polypeptide specific antigen (TPS) in 1992[28,29]. Subsequent to this, monoclonal tissue polypeptide antigen (TPA-M) was developed in 1994. Its utilization has been analyzed with regard to the diagnosis of colorectal adenocarcinoma[30,31] and neoplasms in other organs, such as the prostate[32], ovaries[33], lungs[34], bladder[35] and breast[36].
A comparison between cytokeratins and CEA would be useful for determining whether they have clinical advantages in the diagnosis, staging and prognosis of colon or rectal cancer patients.
A study was made in 169 patients (n) with colorectal adenocarcinoma undergoing surgical treatment. The study was conducted in accordance with international standards (Helsinki Declaration)[37], and approved by the Institutional Ethics Committee. Patients who had a previous neoplasm history were excluded. A control group of 100 individuals was recruited among blood donors at the General Hospital, São Paulo.
The patients were informed that the study period would consist of the surgical phase and a postoperative follow-up period of 6, 12, 18, 24, 36, 48 and 60 mo. The preoperative staging was achieved via clinical evaluation, colonoscopy, computerized tomography (CT) of the abdomen and pelvis, and chest radiography. Opaque enema, nuclear magnetic resonance (NMR) and bone scintigraphy were performed in accordance with the clinical indications of each case.
Blood samples were centrifuged and the peripheral serum was frozen at -20 °C. The patients were periodically followed up during the postoperative period by means of clinical evaluation and performing the examinations mentioned. In the control group, peripheral blood samples were collected via procedures similar to those used for the patients.
With regard to ethnicity, 69.2% of the patients were whites, 20.1% brown-skinned, 7.7% yellow-skinned and 3% blacks. With regard to gender, 43.2% were males. At the time of diagnosis, the average age was 62.2 years, ranging from 19 to 89 years. Fifty-four point four percent of the lesion locations were in the rectum, 18.9% in the left colon, 3.6% in the transverse colon and 23.1% in the right colon. The average diameter of the neoplasms was 6.1 cm, ranging from 1 to 17 cm.
Of the initial 169 patients, 120 underwent curative surgery (71%). The average time of follow-up was 22.3 mo.
Follow-up was lost in 3 patients (1.8%). Of the 120 patients undergoing radical surgery, 81 (67.5%) completed the follow.
Among the 120 patients undergoing radical surgery, 23 (19.2%) presented neoplastic relapse at an average of 18.1 mo after the initial surgery.
The control group was composed of 100 blood donors at the General Hospital, São Paulo (HGSP). Forty-five percent of the donors were whites, 39% brown-skinned, 12% blacks and 4% yellow-skinned. Fifty-four were males. At the time of blood donation, their average age was 42.5 years, ranging from 18 to 60 years.
A single professional at the Clinical Analysis Laboratory of Hospital São Paulo, Federal University of São Paulo - Escola Paulista de Medicina, performed the serum assays for tumor markers. The CEA level was determined via the Delfia® method, using the Cobas Mira Plus® automatic analyzer from Roche®, and the limit for normality was considered to be 5 ng/mL[10,11,18]. The cytokeratin levels were determined via the LIA-mat TPA-M Prolifigen® method from the AB Sangtec Medical® Laboratory, using the Lumat LB 9501® Luminometer from EG&G Berthold, and the reference value of 72 U/L.
The following were utilized in the statistical analysis: ROC curve[38], kappa statistic analysis (κ)[39], variance analysis[40], Student’s t test (t)[41] and survival analysis[41] via the Kaplan-Meier curves. P<0.05 was considered statistically significant.
In the diagnosis of colorectal adenocarcinoma, CEA demonstrated a sensitivity of 56%, a specificity of 95%, a positive predictive value of 94%, a negative predictive value of 50% and an accuracy of 76.8%. TPA-M presented a sensitivity of 70%, a specificity of 96%, a positive predictive value of 97%, a negative predictive value of 66% and an accuracy of 93.6% (Figure 1).
The elevation of one of the markers was shown to have a sensitivity of 76.9%, a specificity of 91%, a positive predictive value of 93.5%, a negative predictive value of 70% and an accuracy of 83.6%. The reagents had independent action modes in samples from the patients.
There was no variation in the levels of the markers according to the degree of cell differentiation while there was an elevation in their concentrations in accordance with the neoplastic dissemination. There was a statistically significant difference between the patients with stage IV lesions and those with stages I, II and III tumors.
With regard to CEA, the average level was 14.2 ng/mL in patients with stage I lesions, 8.5 ng/mL in patients with stage II lesions, 8.0 ng/mL in patients with stage III lesions and 87.7 ng/mL in patients with stage IV lesions. In relation to TPA-M, the levels were 153.1 U/L in patients with stage I tumors, 106.5 U/L in patients with stage II tumors, 136.3 U/L in patients with stage III tumors and 464.3 U/L in patients with stage IV tumors.
The sensitivity of each marker or its association with stages I, II, III and IV of the TNM classification is described in Table 1.
| Sensitivity at different stages (%) | ||||
| I | II | III | IV | |
| CEA | 35 | 23.3 | 34.1 | 69 |
| TPA-M | 75 | 53.3 | 61 | 82.8 |
| Increased CEA or TPA-M | 77.5 | 60 | 68.3 | 91.4 |
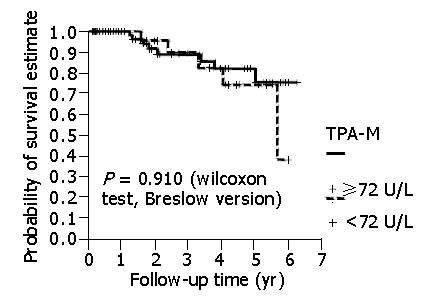
There was a statistical difference in patients with a high CEA level in relation to a shorter survival. However, there was no statistical difference between patients with high TPA-M levels in relation to a shorter survival (Figure 2). Even when higher cut-off values for TPA-M were adopted (216 U/L, three times higher than the maximum value were considered to be normal), no significant differences were discovered.
The exclusion criteria for patients with a prior history of benign or malignant neoplasms were appropriate, because they could have an increase in the levels of the serum tumor markers analyzed not due to the colorectal neoplasm present. For the control group, blood donors present colorectal neoplasm were used as the healthy population sample as in other studies of the same nature[30,31].
CEA presented unsatisfactory results when the diagnosis of colorectal neoplasia was made, with a diagnostic rate of about 40%[10-12]. In this study, the sensitivity was approximately 50%, confirming that this marker should, therefore not be utilized for the diagnosis of lesions[42-44]. The cytokeratins assayed via TPA-M showed a sensitivity of about 70%. Correale et al[30] found a sensitivity of 48% with a cut-off point of 70 U/L. Plebani et al[31] by using TPA-M with a cut-off value of 46 U/L, identified a sensitivity of 58%. The rates obtained in the present investigation appear to be promising. Additional studies are necessary for verifying the real sensitivity of TPA-M in colorectal cancer patients.
Plebani et al[31] foresaw the advantages in utilizing cytokeratins in combination with CEA in the diagnosis of colorectal neoplastic lesions. On the basis of the data from the present research, the utilization of cytokeratins and CEA was attractive, with a sensitivity of 77%.
Perhaps the use of TPA-M in combination with CEA will make it possible to detect colorectal neoplasms in populations at risk at a reasonable cost, especially when its ease of execution and elevated sensitivity are considered.
With such a sensitivity, TPA-M may have some usefulness, even in terms of diagnosis, of the lesions that have the macroscopic characteristics of neoplasm in endoscopic or radiological examinations but without confirmation of the malignant nature from the anatomopathological examination. Increased use of TPA-M in combination with CEA may constitute an additional element for indicating surgical interventions.
The association between quantification of these tumor markers and staging of patients is relative. Carriquiry and Piñeyro [10] studied 209 patients, and identified an average preoperative CEA level of 4.25 ng/mL in patients with stage I tumors, 7.49 ng/mL in patients with stage II tumors, 6.42 ng/mL in patients with stage III tumors, and 241.88 ng/mL in patients with stage IV tumors. The percentage of increased CEA at each stage was 21%, 31%, 36% and 92% in patients with stages I, II, III and IV tumors, respectively.
Correale et al[30] studied TPA-M assays taken from 98 patients with malignant colorectal tumors using a cut-off point of 57 U/L, and found the sensitivity 33%, 35%, 59% and 73% in patients with stages I, II, III, and IV tumors, respectively. Plebani et al[31] reported a statistically significant variation in TPA-M levels only in patients with stage IV colorectal neoplasm, in relation to those with other stage tumors.
In this research, the sensitivity of CEA and TPA-M presented a statistically significant difference between stage IV and the other stage tumors. Larger samples would perhaps be able to find evidence for other differences. However, it is possible that elevation in serum levels of tumor markers might only be provoked by lesions that extend beyond the colon or rectum. In any event, preoperative assay of the markers would show some value in the staging, and should be done for all patients.
Several studies demonstrated lower levels on the survival curves for patients with elevated CEA assays during the preoperative period, such as the studies by Carriquiry and Piñeyro[10], Wang et al[14] and Wiratkapun et al[15].
No studies are available regarding the correlation between preoperative TPA-M levels above or below 72 U/L and prognostic indices such as disease-free intervals and relapse, in patients with colon or rectum cancer.
In the present study, no statistical difference was identified in the disease-free interval and survival of patients with preoperative concentrations of TPA-M above or below the level of 72 U/L. In the same way, with cut-off value three times greater than normal, as calculated by Forones et al[13] and Wiratkapun et al[15] for CEA, there was no difference in patient survival for TPA-M. This does not, however, necessarily signify that there is no difference between the groups of individuals with normal or elevated pre-surgical levels of this tumor marker. It is possible that the patient sample did not have medical follow-up for a sufficient period of time for a statistical difference to emerge between the groups in relation to the marker studied. In this research, the average patient follow-up time was 22.3 mo. Other investigations[10,14,15] did have a longer follow-up, with a statistical difference identified in survival. TPA-M can demonstrate prognostic importance in studies with a longer follow-up time.
The peripheral serum level of CEA during the preoperative period reached the status of a relevant prognostic variable. At present, the preoperative staging of colorectal cancer includes CEA assay with the following classification: CX - undetermined CEA level, C0 - level less than 5 ng/mL, and C1 - level greater than 5 ng/mL[45].
What are the intrinsic characteristics that would define an ideal tumor marker? The level of such a marker would rise in the presence of the smallest neoplastic lesions, and would increase only with the existence of tumors. The marker would be produced by all neoplastic cells, thus making it possible to correlate between marker levels and tumor extent. All patients would generate such a marker. For the public, the examination must have an accessible cost, be minimally invasive and can be performed in any location. The marker should precisely indicate the diagnosis, staging, prognosis and occurrence of neoplastic relapse. There is a consensus on the fact that the ideal tumor marker does not exist[8,46].
Cytokeratins constitute an advance in the direction of a perfect tumor marker, and their association with CEA is useful in offering a better approach towards patients with colorectal cancer.
Edited by Wang XL
| 1. | Hoel DG, Davis DL, Miller AB, Sondik EJ, Swerdlow AJ. Trends in cancer mortality in 15 industrialized countries, 1969-1986. J Natl Cancer Inst. 1992;84:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 64] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Colonna M, Grosclaude P, Launoy G, Tretarre B, Arveux P, Raverdy N, Benhamiche AM, Herbert C, Faivre J. Estimation of colorectal cancer prevalence in France. Eur J Cancer. 2001;37:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Wilmink AB. Overview of the epidemiology of colorectal cancer. Dis Colon Rectum. 1997;40:483-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Wang HZ, Huang XF, Wang Y, Ji JF, Gu J. Clinical features, diagnosis, treatment and prognosis of multiple primary colorectal carcinoma. World J Gastroenterol. 2004;10:2136-2139. [PubMed] |
| 5. | Zinkin LD. A critical review of the classifications and staging of colorectal cancer. Dis Colon Rectum. 1983;26:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 57] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Hurst R, Stamos M, Wilmoth G, Lin C. Rectal carcinoma: are we making a difference? Am Surg. 1996;62:806-810. [PubMed] |
| 7. | Theile DE, Cohen JR, Holt J, Davis NC. Mortality and complications of large-bowel resection for carcinoma. Aust N Z J Surg. 1979;49:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Bates SE. Clinical applications of serum tumor markers. Ann Intern Med. 1991;115:623-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 150] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Gold P, Freedman SO. Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med. 1965;121:439-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1572] [Cited by in RCA: 1544] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 10. | Carriquiry LA, Piñeyro A. Should carcinoembryonic antigen be used in the management of patients with colorectal cancer? Dis Colon Rectum. 1999;42:921-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Li Destri G, Greco S, Rinzivillo C, Racalbuto A, Curreri R, Di Cataldo A. Monitoring carcinoembryonic antigen in colorectal cancer: is it still useful? Surg Today. 1998;28:1233-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Engarås B, Kewenter J, Nilsson O, Wedel H, Hafström L. CEA, CA 50 and CA 242 in patients surviving colorectal cancer without recurrent disease. Eur J Surg Oncol. 2001;27:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Forones NM, Tanaka M, Falcão JB. CEA as a prognostic index in colorectal cancer. Sao Paulo Med J. 1997;115:1589-1592. [PubMed] |
| 14. | Wang WS, Lin JK, Chiou TJ, Liu JH, Fan FS, Yen CC, Lin TC, Jiang JK, Yang SH, Wang HS. Preoperative carcinoembryonic antigen level as an independent prognostic factor in colorectal cancer: Taiwan experience. Jpn J Clin Oncol. 2000;30:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Wiratkapun S, Kraemer M, Seow-Choen F, Ho YH, Eu KW. High preoperative serum carcinoembryonic antigen predicts metastatic recurrence in potentially curative colonic cancer: results of a five-year study. Dis Colon Rectum. 2001;44:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Steele G, Zamcheck N, Wilson R, Mayer R, Lokich J, Rau P, Maltz J. Results of CEA-initiated second-look surgery for recurrent colorectal cancer. Am J Surg. 1980;139:544-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Minton JP, Hoehn JL, Gerber DM, Horsley JS, Connolly DP, Salwan F, Fletcher WS, Cruz AB, Gatchell FG, Oviedo M. Results of a 400-patient carcinoembryonic antigen second-look colorectal cancer study. Cancer. 1985;55:1284-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Lucha PA, Rosen L, Olenwine JA, Reed JF, Riether RD, Stasik JJ, Khubchandani IT. Value of carcinoembryonic antigen monitoring in curative surgery for recurrent colorectal carcinoma. Dis Colon Rectum. 1997;40:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Wichmann MW, Müller C, Lau-Werner U, Strauss T, Lang RA, Hornung HM, Stieber P, Schildberg FW. The role of carcinoembryonic antigen for the detection of recurrent disease following curative resection of large-bowel cancer. Langenbecks Arch Surg. 2000;385:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Nakayama T, Watanabe M, Teramoto T, Kitajima M. CA19-9 as a predictor of recurrence in patients with colorectal cancer. J Surg Oncol. 1997;66:238-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Nilsson O, Johansson C, Glimelius B, Persson B, Nørgaard-Pedersen B, Andrén-Sandberg A, Lindholm L. Sensitivity and specificity of CA242 in gastro-intestinal cancer. A comparison with CEA, CA50 and CA 19-9. Br J Cancer. 1992;65:215-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Fernández-Fernández L, Tejero E, Tieso A. Significance of CA 72-4 in colorectal carcinoma. Comparison with CEA and CA 19-9. Eur J Surg Oncol. 1995;21:388-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Björklund B. Tissue polypeptide antigen (TPA): Biology, biochemistry, improved assay methodology, clinical significance in cancer and other conditions, and future outlook. Antibiot Chemother (1971). 1978;22:16-31. [PubMed] |
| 24. | Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3859] [Cited by in RCA: 3874] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 25. | Broll R, Erdmann H, Duchrow M, Oevermann E, Schwandner O, Markert U, Bruch HP, Windhövel U. Vascular endothelial growth factor (VEGF)--a valuable serum tumour marker in patients with colorectal cancer? Eur J Surg Oncol. 2001;27:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Shiota G, Ishida M, Noguchi N, Oyama K, Takano Y, Okubo M, Katayama S, Tomie Y, Harada K, Hori K. Circulating p53 antibody in patients with colorectal cancer: relation to clinicopathologic features and survival. Dig Dis Sci. 2000;45:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Hammel P, Soussi T. Serum p53 antibody assay: evaluation in colorectal cancer. Rev Med Interne. 2000;21:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Lindmark G, Bergström R, Påhlman L, Glimelius B. The association of preoperative serum tumour markers with Dukes' stage and survival in colorectal cancer. Br J Cancer. 1995;71:1090-1094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Carpelan-Holmström M, Haglund C, Lundin J, Alfthan H, Stenman UH, Roberts PJ. Independent prognostic value of preoperative serum markers CA 242, specific tissue polypeptide antigen and human chorionic gonadotrophin beta, but not of carcinoembryonic antigen or tissue polypeptide antigen in colorectal cancer. Br J Cancer. 1996;74:925-929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Correale M, Arnberg H, Blockx P, Bombardieri E, Castelli M, Encabo G, Gion M, Klapdor R, Martin M, Nilsson S. Clinical profile of a new monoclonal antibody-based immunoassay for tissue polypeptide antigen. Int J Biol Markers. 1994;9:231-238. [PubMed] |
| 31. | Plebani M, De Paoli M, Basso D, Roveroni G, Giacomini A, Galeotti F, Corsini A. Serum tumor markers in colorectal cancer staging, grading, and follow-up. J Surg Oncol. 1996;62:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 32. | Lewenhaupt A, Ekman P, Eneroth P, Nilsson B, Nordström L. Tissue polypeptide antigen (TPA) as a prognostic aid in human prostatic carcinoma. Prostate. 1985;6:285-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Panza N, Pacilio G, Campanella L, Peluso G, Battista C, Amoriello A, Utech W, Vacca C, Lombardi G. Cancer antigen 125, tissue polypeptide antigen, carcinoembryonic antigen, and beta-chain human chorionic gonadotropin as serum markers of epithelial ovarian carcinoma. Cancer. 1988;61:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Gronowitz JS, Bergström R, Nôu E, Påhlman S, Brodin O, Nilsson S, Källander CF. Clinical and serologic markers of stage and prognosis in small cell lung cancer. A multivariate analysis. Cancer. 1990;66:722-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Tizzani A, Casetta G, Cavallini A, Piana P, Piantino P. Blood and urine determinations of tissue polypeptide antigen in patients with bladder carcinoma. Minerva Urol Nefrol. 1990;42:69-71. [PubMed] |
| 36. | Barak M, Steiner M, Finkel B, Abrahamson J, Antal S, Gruener N. CA-15.3, TPA and MCA as markers for breast cancer. Eur J Cancer. 1990;26:577-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Annas GJ. The changing landscape of human experimentation: Nuremberg, Helsinki, and beyond. Health Matrix Clevel. 1992;2:119-140. [PubMed] |
| 38. | Fletcher RH, Fletcher SW, Wagner EW. Epidemiologia Clínica. Porto Alegre:. Artes Médicas. 1989;. |
| 39. | Agresti A. Categorical Data Analysis. New York: Wiley Interscience 1990; . |
| 40. | Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied linear statistical models. 4th ed. Boston: Irwin 1996; . |
| 41. | Kalbfleisch JD, Prentice RL. The statistical analysis of time failure data. New York: John Wiley and Sons 1980; . |
| 42. | Fletcher RH. Carcinoembryonic antigen. Ann Intern Med. 1986;104:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 351] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 43. | Trillet-Lenoir V, Freyer G. Advantage of using tumor markers in colorectal and breast cancers. Guidelines of the American Society of Clinical Oncology (ASCO). Bull Cancer. 1997;84:767-768. [PubMed] |
| 44. | Bast RC, Ravdin P, Hayes DF, Bates S, Fritsche H, Jessup JM, Kemeny N, Locker GY, Mennel RG, Somerfield MR. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1865-1878. [PubMed] |
| 45. | Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer. 2000;88:1739-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 46. | Fernandes LC, Matos D. Marcadores tumorais no câncer colorretal. Rev Col Bras Cir. 2002;29:106-111. |