Published online Dec 28, 2005. doi: 10.3748/wjg.v11.i48.7597
Revised: July 3, 2005
Accepted: July 8, 2005
Published online: December 28, 2005
AIM: To evaluate the interplay between gliadin and LoVo cells and the direct effect of gliadin on cytoskeletal patterns.
METHODS: We treated LoVo multicellular spheroids with digested bread wheat gliadin in order to investigate their morphology and ultrastructure (by means of light microscopy and scanning electron microscopy), and the effect of gliadin on actin (phalloidin fluorescence) and the tight-junction protein occludin and zonula occluden-1.
RESULTS: The treated spheroids had deep holes and surface blebs, whereas the controls were smoothly surfaced ovoids. The incubation of LoVo spheroids with gliadin decreased the number of intracellular actin filaments, impaired and disassembled the integrity of the tight-junction system.
CONCLUSION: Our data obtained from an "in vivo-like" polarized culture system confirm the direct noxious effect of gliadin on the cytoskeleton and tight junctions of epithelial cells. Unlike two-dimensional cell culture systems, the use of multicellular spheroids seems to provide a suitable model for studying cell-cell interactions.
-
Citation: Dolfini E, Roncoroni L, Elli L, Fumagalli C, Colombo R, Ramponi S, Forlani F, Bardella MT. Cytoskeleton reorganization and ultrastructural damage induced by gliadin in a three-dimensional
in vitro model. World J Gastroenterol 2005; 11(48): 7597-7601 - URL: https://www.wjgnet.com/1007-9327/full/v11/i48/7597.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i48.7597
Celiac disease (CD) is an immunomediated intestinal disorder that is triggered by dietary gluten and related cereal proteins in genetically susceptible individuals[1,2]. Gluten consists of a complex mixture of gliadin monomers and large polymeric glutenin polypetides. A number of in vitro studies of two-dimensional cell cultures have shown that gliadin has direct cytotoxic effects on epithelial cells[3], but the early steps allowing the start of the immunoreaction are largely unknown.
CD is characterized by enhanced paracellular permeability across the intestinal epithelium, a “leaky gut” condition that allows the passage of macromolecules through the paracellular spaces[4-6]. Moreover, it has also been demonstrated that cytoskeleton involved in the pathogenesis of CD as a gluten challenge rapidly causes the disappearance and disorganization of actin filaments in the intestinal mucosa of CD patients[7]. The actin filaments in epithelial cells are associated with tight junctions (TJs), appearing as a series of discrete sites of apparent membrane fusion (so called “kissing points”) involving the outer leaflets of the plasma membranes of adjacent cells. The integrity of the barrier function is important for the separation of two different compartments, and TJs play a major role in controlling paracellular transport between the luminal and basolateral fluid compartments[8].
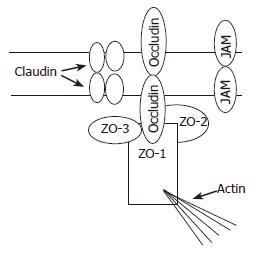
Almost all the proteins associated with TJs are peripheral membrane proteins that form part of the submembrane plaque (Figure 1). The first TJ-associated protein to be identified is zonula occluden-1 (ZO-1) whose C-terminal half contains an actin-binding site and mediates interactions between transmembrane proteins and cytoskeleton elements[9]. Occludin, a 60 ku integral membrane protein in TJ strands[10], is involved in TJ barrier and fence functions through its four transmembrane domains, three cytoplasmic domains and two extracellular loops[8]. Claudin-1 and claudin-2 are 23 ku integral membrane proteins that function as major structural components of TJ strands[11]. Junctional adhesion molecules (JAMs) have only one putative transmembrane sequence. The intracellular domain consists of 45 residues, and the extracellular portion (215 residues) contains two domains with intra chain disulfide bonds[12].
The cytoskeletal network generates tension and transmits stress within and among the cells[13]. The formation of multicellular tumor spheroids (MCTSs) involves cell translocations and morphological changes that are indicative of the organization of the cytoskeleton. The cells grown in MCTSs are interconnected by the means of TJs that form a seal between adjacent cells, thus defining their apical and basolateral surfaces, and creating a model similar to in vivo tissue. This is very different from the organization of two-dimensional cell cultures[14,15].
The aim of this study was to evaluate the interplay between gliadin and LoVo MCTSs, with specific emphasis on the direct effect of gliadin on cytoskeleton patterns and reorganization.
Cells from the human colon adenocarcinoma cell line (LoVo, ATCC, Rockville, USA) were grown in T75 flasks (PBI, Italy) at 37 °C in an atmosphere containing 95% air and 50 mL/L CO2. The medium consisted of Ham’s F-12 medium (GIBCO, Italy), supplemented with 10% fetal bovine serum (GIBCO, Italy), 1% MEM vitamin solution 100× (GIBCO, Italy), and 3% L-glutamine 200 mmol/L (GIBCO, Italy).
After one week, the cells were removed using solution containing 0.25% (w/v) trypsin and 0.02% (w/v) EDTA (Sigma-Aldrich, Italy), and the cell suspensions were cultured again. Mycoplasma contamination was regularly searched for and excluded using the Hoechst method[16].
Gliadin was purified from Triticum aestivum flour (Hereward Cultivar, UK) according to Capelli[17]. Pepsin (3.2-4.5 U/mg) was supplied by Sigma (Italy), and the pancreatin (0.1 mAnson/mg) by Merck (USA). All the chemicals were of analytical grade. Digestion was performed as previously described by our group[18]. Briefly, the gliadin was first incubated with pepsin at 37 °C for 24 h, and then with pancreatin at 37 °C for 3 h, adjusting to a pH of 8. The digested protein was analytically controlled by means of RP-HPLC, SE-HPLC, and SDS-PAGE, freeze-dried and stored.
Three-dimensional cell cultures were initiated by seeding 4×105 cells/mL in 25 mL of complete medium supplemented with penicillin (100 U/mL) and streptomycin (100 Ug/mL) (GIBCO, Italy) in Erlenmeyer flasks (Corning, Italy), and incubated in a gyratory rotation incubator (60 rev/min) at 37 °C in air (Colaver, Italy). Homotypical aggregations were visible after 4 d of culture, and the MCTSs were usually complete within 7 d (average diameter±SD, 370±48.5 μm).
On the seventh day, the MCTSs were exposed to PT-digested gliadin (500 μg/mL) in a completely renewed medium for further 4 d and subsequently taken for microscopic examination. The PT-digested gliadin greatly inhibited cell growth (50% inhibitory concentration: 390 μg/mL) and the dose used was selected from four different concentrations (125, 500, 750, 1 000 μg/mL) on the basis of previous data obtained in our laboratory[18,19].
Three-dimensional cell cultures were washed twice in PBS, and then fixed in 2.5% glutaraldehyde in phosphate buffer at room temperature for 24 h at 4 °C. At the time of analysis, a representative sample of spheroids was recovered, immediately placed on a paper filter and observed in low vacuum modality at a high voltage of 10 kV. SEM analysis was performed using a Philips Scanning Electron Microscope (Mod. ×L20).
LoVo MCTSs were washed twice in PBS, fixed in 4% paraformaldeyde for 1 h, permeabilized with 0.4% Triton X-100 (Sigma-Aldrich, Italy) for 20 min, washed thrice for 5 min in PBS and stained for immunocytochemistry by means of incubation with fluorescein TRITC-phalloidin (Sigma-Aldrich, Italy) (1:200 PBS) in a humid chamber at room temperature for 6 h. After washing thrice in PBS each for 5 min, 10 spheroids were transferred onto slides, and each slide was mounted with 90% glycerol in PBS. The results were analyzed using a confocal laser scanning microscope (Leica TCSNT, Germany).
LoVo MCTSs were washed twice in PBS and fixed in ethanol for 30 min at 4 °C. After the first incubation, the samples were incubated with acetone (previously stored at -20 °C) for an additional 3 min at room temperature. They were then blocked and incubated for immunocytochemistry overnight with anti-occludin-FITC (Zymed, CA, USA) before being analyzed by means of confocal laser scanning microscopy (Leica TCSNT, Germany).
LoVo MCTSs were washed twice in PBS and fixed in ethanol for 30 min at 4 °C. After the first incubation, the samples were incubated with acetone (previously stored at -20 °C) for an additional 3 min at room temperature. They were then blocked and incubated overnight with anti-ZO-1-FITC (Zymed, CA, USA) before being analyzed by means of confocal laser scanning microscopy (Leica TCSNT, Germany).
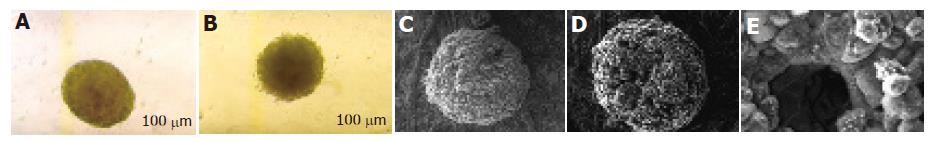
The untreated MCTSs appeared bright and round at phase-contrast microscopy (Figure 2A). SEM showed that they were well-defined and compact, with smooth boundaries and a regular surface. Their structure was compact, densely organized and tightly packed (Figure 2C).
The MCTSs treated with PT-digested gliadin were loosely connected and irregularly shaped (Figure 2B). They were less bright than the controls and had frayed borders. SEM revealed an altered surface, with deep and irregularly distributed holes (Figures 2D and E).
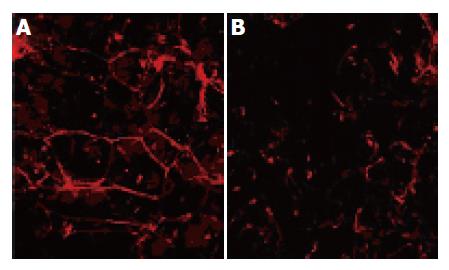
The untreated MCTSs stained with TRITC-phalloidin had regular perijunctional actin rings and showed organized distribution at the cell boundaries (Figure 3A), whereas those treated with PT-digested gliadin had reorganized intracellular actin filaments and disassembled F-actin (Figure 3B).
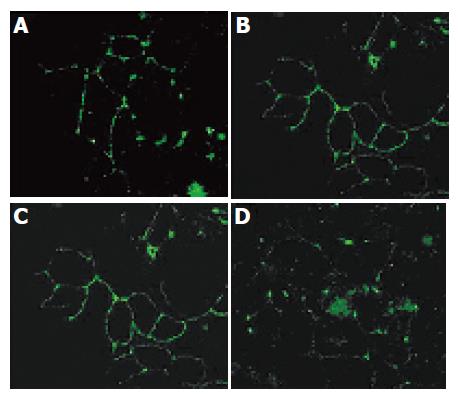
In comparison with the normal subapical honeycomb pattern typical of TJs (Figures 4A and C), treatment with PT-digested gliadin led to their structural dissociation (Figures 4B and D). The morphologically characteristic ring structure of occludin and ZO-1 immunolocalization in the en face confocal images had sharp boundaries in the untreated spheroids, but was partially or completely lost in the treated spheroids.
CD is a chronic intestinal inflammatory disorder characterized by mucosal changes including lymphocyte infiltration, crypt hyperplasia and villous atrophy. Furthermore, intestinal permeability is increased and TJs appear open[20]. As TJs form a barrier against the diffusion of molecules from the lumen to the tissue parenchyma (barrier function), and restrict the diffusion of lipids and proteins between the apical and basolateral plasma membranes (fence function)[21], the loss of permeability allows the translocation of antigenic molecules from the intestinal lumen to the lamina propria, thus creating a condition for an immune response.
Traditional tissue culture methods have been based on growing cell lines as monolayers, but a new three-dimensional culture system (Figure 2A) has been investigated as a mean of modeling a solid tumor under in vitro conditions that simulates its in vivo biological properties. Given the fundamental differences between monolayer and three-dimensional cultures, spheroids should become mandatory test systems in oncological therapeutic screening programs[22]. Furthermore, cells grown in three-dimensional cultures are oriented and polarized[23,24], and often express a gene repertoire that is different from that of the monolayer cultures[25]. Previous studies from our laboratory have confirmed the polarized structure, and the presence of microvilli and tightly connected epithelial junctional complexes[18].
The interaction between TJ proteins and actomyosin cytoskeleton in MCTSs is a primary target for physiological and pathological signals. Circumferential actomyosin contraction and cytoskeleton interaction modulate TJ permeability, thus contributing to the formation of the TJ fence[21]. Pizzuti et al[26]. have recently demonstrated that the intestinal mucosa of celiac patients has an altered TJ system during gluten exposure, and decreased ZO-1 expression is associated with a disrupted F-actin organization and the loss of normal distribution at cell-cell contact sites.
Our SEM morphological examinations confirmed that gliadin treatment could affect spheroid structure, causing a loss of cell thickness and organization, and the formation of hole-like surface structures (Figures 2C-E). Confocal laser scanning microscopy showed that gliadin could induce cytoskeleton reorganization (Figures 3A and B), and specifically act on TJ structural proteins.
Though our results have confirmed that the expression of occludin correlates with barrier properties, the decreased intensity or disappearance of occludin staining at cell–cell borders in gliadin-treated spheroids (Figures 4A and B) do not presuppose the absence of TJ structural integrity. Knockout experiments in mouse embryonic stem cells have demonstrated that occludin is not necessary to form functionally competent TJs[27], and ZO-1 has a wild-type localization in apical junctional regions of the outermost layer of epithelial cells in the absence of occludin[28]. We found that the immunolocalization of ZO-1 in TJ ring structures was severely disrupted by gliadin exposure, leading to almost complete lack of continuity, and en face confocal images showed a breakdown of junctional complexes (Figures 4C and D). Taken together, these findings suggest that ZO-1 lies at the centre of a network of protein–protein interactions and may be critical in recruiting the proteins necessary to establish TJs.
Our preliminary data showed that TJ permeability might be regulated directly as a result of TJ protein modifications, or indirectly as a result of the effects of xenobiotics on the cytoskeleton. New insights into the molecular architecture of TJs and their regulation have given rise to a new concept of TJ modulation based on peptides from the first extracellular domain of occludin[29]. We can speculate that gliadin peptides also act as modulators on the extracellular loops of TJ transmembrane proteins, mediating TJ opening and the consequent cytoskeletal redistribution. According to the “tensegrity model”[30,31], the cells are in prestressed structures in which cytoskeletal elements are major determinants of deformability, and so a local stress can cause global structural rearrangements. The cell response to xenobiotic exposure leads to the retraction of submembranous actin filaments from junctional complexes, thus determining the disappearance of organized TJs. Furthermore, this alteration of internal balance of tensile stress compromises the stability of cell shape: the cells become rounded and stimulate an apoptotic process. The correlation of apoptosis with a disrupted cytoskeleton and junctional system may explain the SEM images: the blebs and holes visible on the surface of treated spheroids are the results of an apoptotic process initiated by the deregulation of internal cell balance. Ojakian et al[23]. reported that gliadin has an apoptotic effect on Caco-2 colon carcinoma cells directly stimulated by digested gliadin.
Understanding the events of this process will throw light onto the changes in paracellular permeability caused by gliadin and identify novel therapeutic targets in celiac disease. Though the intricacies of this process in vivo have not yet been fully elucidated, our results indicate that a pivotal role is played by the disarrangement of the “belt-like” structure of perijunctional F-actin affiliated with TJs. This highlights the importance of the cytoskeleton network in the ultrastructural architecture of enterocytes, given that a gluten challenge in CD patients rapidly distorts the microvillous structure, thus disorganizing the actin network on the intestinal mucosa.
If the early steps of gliadin-induced mucosal damage in patients with CD concern intestinal permeability, which is directly altered by gliadin before the immunological response, MCTSs could become essential for testing the cytotoxic effects of new chemically, enzymatically or genetically modified gliadins studied as alternative therapies to a gluten-free diet.
The authors would like to thank Kevin Smart (Link srl, Milan), “Centro per lo Studio della Celiachia” of the University of Milan, for his help in preparing the English version of the manuscript, Maria Letizia Falini and Rosita Caramanico for their help in preparing peptides, and San Paolo Bank for financial support.
The study was conceived and designed by E. Dolfini, R. Colombo, L. Roncoroni, C. Fumagalli, L. Elli, and M.T. Bardella. L. Roncoroni and C. Fumagalli were responsible for the cell cultures. V. Lorusso and S. Ramponi undertook the morphological investigations and F. Forlani prepared the digested gliadin.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology. 2001;120:636-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 737] [Article Influence: 30.7] [Reference Citation Analysis (1)] |
| 2. | Green PH, Jabri B. Coeliac disease. Lancet. 2003;362:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 593] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 3. | Elli L, Dolfini E, Bardella MT. Gliadin cytotoxicity and in vitro cell cultures. Toxicol Lett. 2003;146:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Cooper BT. Intestinal permeability in coeliac disease. Lancet. 1983;1:658-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Bjarnason I, Peters TJ, Veall N. Intestinal permeability defect in coeliac disease. Lancet. 1983;1:1284-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Schulzke JD, Bentzel CJ, Schulzke I, Riecken EO, Fromm M. Epithelial tight junction structure in the jejunum of children with acute and treated celiac sprue. Pediatr Res. 1998;43:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 129] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Sjölander A, Magnusson KE. Effects of wheat germ agglutinin on the cellular content of filamentous actin in Intestine 407 cells. Eur J Cell Biol. 1988;47:32-35. [PubMed] |
| 8. | Tsukita S, Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 420] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 9. | Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755-766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1148] [Cited by in RCA: 1226] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 10. | Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777-1788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1844] [Cited by in RCA: 1874] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 11. | Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539-1550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1524] [Cited by in RCA: 1572] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 12. | Martìn-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1051] [Cited by in RCA: 992] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 13. | Bates RC, Buret A, van Helden DF, Horton MA, Burns GF. Apoptosis induced by inhibition of intercellular contact. J Cell Biol. 1994;125:403-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 184] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Conforti G, Codegoni AM, Scanziani E, Dolfini E, Dasdia T, Calza M, Caniatti M, Broggini M. Different vimentin expression in two clones derived from a human colocarcinoma cell line (LoVo) showing different sensitivity to doxorubicin. Br J Cancer. 1995;71:505-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Matsubara S, Ozawa M. Expression of alpha-catenin in alpha-catenin-deficient cells results in a reduced proliferation in three-dimensional multicellular spheroids but not in two-dimensional monolayer cultures. Oncogene. 2004;23:2694-2702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | La Porta CA, Dolfini E, Comolli R. Inhibition of protein kinase C-alpha isoform enhances the P-glycoprotein expression and the survival of LoVo human colon adenocarcinoma cells to doxorubicin exposure. Br J Cancer. 1998;78:1283-1287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Capelli L, Forlani F, Perini F, Guerrieri N, Cerletti P, Righetti PG. Wheat cultivar discrimination by capillary electrophoresis of gliadins in isoelectric buffers. Electrophoresis. 1998;19:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Dolfini E, Elli L, Ferrero S, Braidotti P, Roncoroni L, Dasdia T, Falini ML, Forlani F, Bardella MT. Bread wheat gliadin cytotoxicity: a new three-dimensional cell model. Scand J Clin Lab Invest. 2003;63:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 19. | Dolfini E, Elli L, Dasdia T, Bufardeci B, Colleoni MP, Costa B, Floriani I, Falini ML, Guerrieri N, Forlani F. In vitro cytotoxic effect of bread wheat gliadin on the LoVo human adenocarcinoma cell line. Toxicol In Vitro. 2002;16:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Clemente MG, De Virgiliis S, Kang JS, Macatagney R, Musu MP, Di Pierro MR, Drago S, Congia M, Fasano A. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut. 2003;52:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 255] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 21. | Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213-C1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1008] [Cited by in RCA: 1042] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 22. | Desoize B. Contribution of three-dimensional culture to cancer research. Crit Rev Oncol Hematol. 2000;36:59-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Ojakian GK, Schwimmer R. Regulation of epithelial cell surface polarity reversal by beta 1 integrins. J Cell Sci. 1994;107:561-576. [PubMed] |
| 24. | Laderoute KR, Murphy BJ, Short SM, Grant TD, Knapp AM, Sutherland RM. Enhancement of transforming growth factor-alpha synthesis in multicellular tumour spheroids of A431 squamous carcinoma cells. Br J Cancer. 1992;65:157-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Murphy BJ, Laderoute KR, Vreman HJ, Grant TD, Gill NS, Stevenson DK, Sutherland RM. Enhancement of heme oxygenase expression and activity in A431 squamous carcinoma multicellular tumor spheroids. Cancer Res. 1993;53:2700-2703. [PubMed] |
| 26. | Pizzuti D, Bortolami M, Mazzon E, Buda A, Guariso G, D'Odorico A, Chiarelli S, D'Incà R, De Lazzari F, Martines D. Transcriptional downregulation of tight junction protein ZO-1 in active coeliac disease is reversed after a gluten-free diet. Dig Liver Dis. 2004;36:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Inazawa J, Fujimoto K, Tsukita S. Mammalian occludin in epithelial cells: its expression and subcellular distribution. Eur J Cell Biol. 1997;73:222-231. [PubMed] |
| 28. | Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol. 2004;36:1206-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 408] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 29. | Tavelin S, Hashimoto K, Malkinson J, Lazorova L, Toth I, Artursson P. A new principle for tight junction modulation based on occludin peptides. Mol Pharmacol. 2003;64:1530-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Ingber DE. Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci. 2003;116:1157-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 913] [Cited by in RCA: 725] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 31. | Ingber DE. Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci. 2003;116:1397-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 518] [Article Influence: 23.5] [Reference Citation Analysis (0)] |