Published online Nov 28, 2005. doi: 10.3748/wjg.v11.i44.7040
Revised: August 8, 2004
Accepted: August 12, 2004
Published online: November 28, 2005
AIM: To establish the cell survival curve for primary hepatic carcinoma cells and to study the relationship between SF2 of primary hepatic carcinoma cells and radiosensitivity.
METHODS: Hepatic carcinoma cells were cultured in vitro using 39 samples of hepatic carcinoma at stages II-IV. Twenty-nine samples were cultured successfully in the fifth generation cells. After these cells were radiated with different dosages, the cell survival ratio and SF2 were calculated by clonogenic assay and SF2 model respectively. The relationship between SF2 and the clinical pathological feature was analyzed.
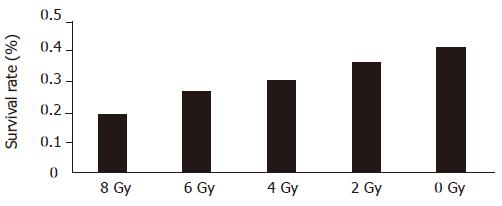
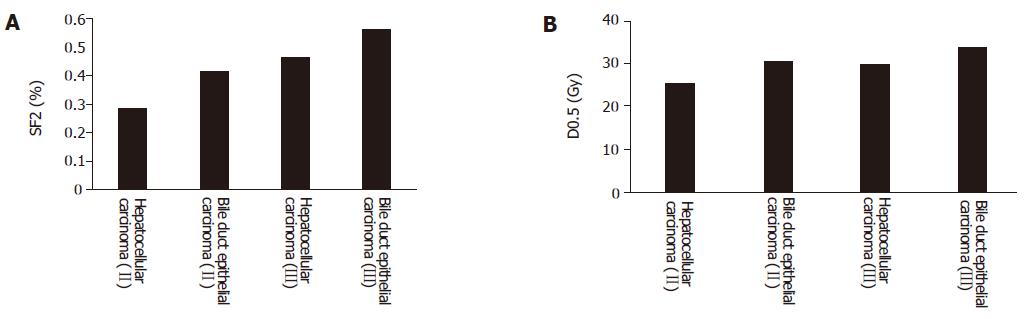
RESULTS: Twenty-nine of thirty-nine samples were successfully cultured. After X-ray radiation of the fifth generation cells with 0, 2, 4, 6, 8 Gy, the cell survival rate was 41%, 36.5%, 31.0%, 26.8%, and 19%, respectively. There was a negative correlation between cell survival and irradiation dosage (r = -0.973, P<0.05). SF2 ranged 0.28-0.78 and correlated with the clinical stage and pathological grade of hepatic carcinoma (P<0.05). There was a positive correlation between SF2 and D0.5 (r = 0.773, P<0.05).
CONCLUSION: SF2 correlates with the clinical stage and pathological grade of hepatic carcinoma and is a marker for predicting the radiosensitivity of hepatic carcinomas.
- Citation: Liu ZZ, Huang WY, Lin JS, Li XS, Lan X, Cai XK, Liang KH, Zhou HJ. Cell survival curve for primary hepatic carcinoma cells and relationship between SF2 of hepatic carcinoma cells and radiosensitivity. World J Gastroenterol 2005; 11(44): 7040-7043
- URL: https://www.wjgnet.com/1007-9327/full/v11/i44/7040.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i44.7040
Hepatic carcinoma is the most common malignant tumors in China. Radiotherapy is its main therapy. But owing to the influence of radiosensitivity and other factors, the effect of radiotherapy on hepatic carcinoma is not obvious. About 40% of hepatic carcinoma patients do not respond to radiotherapy.
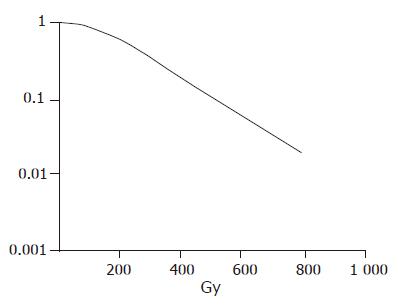
The cell survival curve is a curve that describes the relationship between radiation dose and survival cells.
In general, the proliferation ability of survival cells decreases with the increasing of radiation dose. Detecting the SF2 is a reliable index to predict the radiosensitivity of tumors.
In this experiment, we used the primary cell culture and the clone-formation technique to establish the reliable cell survival curve for hepatic carcinoma. By application of the multi-target click model and calculation of SF2, we studied the relationship between SF2 and D0.5. The value of SF2 for the prediction of the prognosis of hepatic carcinoma was evaluated.
Thirty-nine fresh specimens were taken from hepatic carcinoma patients at stages II-IV. All the patients had their final diagnosis in Tongji Hospital and did not receive any therapy. Their average age was 49.6 years (22-76 years). All the cases were diagnosed by pathology. Twenty-four specimens of hepatocellular carcinoma and 15 samples of bile duct epithelial carcinoma were taken. Nine were in stage IIB (23%), 25 in stage IIIB (64.1%) and 5 in stage IV (12.9%). The maximum diameter of local carcinoma was <4 cm in 22 specimens (56.9%) and >4 cm in 17 specimens (43.1%). The specimens were put into RPMI-1640 medium containing 25% fetal calf serum.
RPMI-1640 medium, bovine trypsin (1:250), insulin, poly-lysine, and hydrocortisone were purchased from JingMei Company, Shenzhen, China. Agarose was purchased from DIFCO Company.
The specimens were irradiated by 6 MV X-ray at the doses of 0, 2, 4, 6, and 8 Gy. The irradiation field was 10 cm×10 cm. The source skin distance (SSD) was 100 cm. The absorbed dose was 200 cGy/min. The culture bottle was covered with 1.5-cm lucite plate.
The specimens were rinsed 2-3 times with PBS containing streptomycin and penicillin. Blood, fibrous and necrosed tissue were removed. Then a small quantity of PBS was added and the tissue was cut into micro-blocks. The micro-blocks of tissue and PBS were left in the plate and incubated in a 50 mL/L CO2 incubator. The plate was kept in inversion status for 4-6 h. The anchoring condition of cells was observed under a microscope. If the cells in the tissue were in good condition, RPMI-1640 medium containing 15-25% fetal calf serum was added. The anchoring condition of cells was observed under a microscope on the next day and poly-lysine was added. The poly-lysine promoted the anchoring of cells. In order to provide the cells a better growing condition, we could also add some stimulation factors such as hydrogenated cortadren (4 μg/mL) and insulin (4 μg/mL) on the third and fourth culture days. The culture medium was changed after 5 d, and then every 2-3 d. After incubation for about 14 d, the cultured cells were digested for future generation.
The fifth generation of hepatic carcinoma cells in logarithmic growth phase was digested by 0.25% trypsinogen and counted. Then the cells were inoculated into cell suspension containing 35% soft agarose. According to the irradiation dosage, the cell suspension was put into glass plates. The culture medium was changed after irradiation for about 12 h. The inoculated cells were divided into five groups and irradiated, and then cultured for 10-14 h. The surface culture medium of the plate was stained with hematoxylin and observed under invert microscope and the number of colonies was more than 50. The clone formation rate (SF) after irradiation was assayed thrice to obtain the mean value.
Three petri dishes were selected from each dosage group to obtain the mean value. O Gy group was used in every experiment and the colony formation rate (SF) was calculated. On the basis of different dosage irradiation and different incubation time, the cell number was used as the ordinate and the irradiation dosage as the abscissa to draw the growth curve for hepatic carcinoma cells. SF2 was calculated by the formula [SF = 1-(1-e-D/Do)N] (the multi-target click model)[2].
The patients were examined once a week before and during radiotherapy. The stage plate was used to measure the size of carcinoma. The volume of tumor was calculated to describe the volume-dosage curve and the dosage decreasing tumor size of 50% (D0.5) was calculated.
The fibroblasts were diffusely distributed in petri dishes after the culture medium was changed before one week. A small quantity of colony formation was also seen. The cell distribution in the center of colony was close. The surrounding cells were distributed outward. The cell density decreased. Different cell colonies had different sizes. Only a few cells could be seen in some new colonies. After 14 d, the fibroblasts in petri dishes increased and their diameter became bigger. The cell distribution in the center of the colony was dense. The diameter of the biggest colony was 8 mm.
Student’s t test was used for the statistical analysis using SPSS 10.0.
After hepatic carcinoma tissue was primarily cultured for about 14-20 d, primary cell culture technique was established in 29 of 39 samples.
The survival curve was drawn after different dosage irradiation of hepatic carcinoma cells. The hepatic carcinoma cells were moderately radiosensitive cells (Figure 1).
The cell survival rate was 41%, 36.5%, 31.0%, 26.8%, and 19%, respectively, after irradiated at the dose of 0, 2, 4, 6, and 8 Gy, respectively. The survival rate of hepatic carcinoma cells had a significantly negative relationship with the irradiation dosage (r = -0.973, P<0.05, Figure 2).
SF2 of hepatic carcinoma cells was 0.28-0.78, which was related with the clinical stage of hepatic carcinoma. The more advanced the clinical stage was, the higher the SF2. SF2 was also related with the pathological typing of hepatic carcinoma. SF2 of bile duct epithelial carcinoma was higher than that of hepatocellular carcinoma (Table 1).
| Clinical stage | |||
| IIb | IIIb | IV | |
| Pathology typing | |||
| Hepatocellular carcinoma | 0.28 | 0.47 | 0.61 |
| Bile duct epithelial carcinoma | 0.41 | 0.57 | 0.78 |
SF2 had a positive relationship with D0.5 (r = 0.773, P<0.05). SF2 in different clinical stages of hepatic carcinoma was also related with D0.5. SF2 in different pathological types of hepatic carcinoma was correlated with D0.5 (Figures 3A and 3B).
The determination of radiosensitivity for tumor cells is an important component of tumor radiobiology. The final objective of tumor radiotherapy is to eradicate tumor. But the objective of clinical radiotherapy is to prevent tumor from growth and to eradicate the tumor. Based on the definition of cell survival, the judge of radiotherapy result is based on whether the cells proliferate or not. The survived cells are the main factor for the failure of radiotherapy[3].
Radiosensitivity of hepatic carcinoma cells is a main factor influencing the prognosis of radiotherapy for hepatic carcinoma. To predict the radiosensitivity of hepatic carcinoma cells before and during the therapy, individualization of reasonable and effective therapy is always the direction of tumor investigation.
Primary tumor cell culture in vitro has some difficulties such as longer time consumption, high cost, and easy contamination. Based on the past experience, the chief key point to the success of this technique is to draw the materials from tissue because it is closely related with the culture. Aseptic technique is one of the important factors for the success of culture.
It was reported that radiosensitivity is different in different hepatic carcinoma cells and that the local control rate of hepatic carcinoma with its SF2 <0.55 is higher than that with its SF2 >0.05[4,5]. SF2 is related with the staging and pathological typing of tumor but not related with the age of patients and the diameter of tumor. SF2 is also correlated with pathological typing and clinical stage of tumor. SF2 for hepatic carcinoma cells in different clinical pathological typing and clinical stage has some differences. It is due to the necessity for individualized treatment of hepatic carcinoma in different clinical pathological typing and clinical stages.
The reports regarding the relationship between SF2 and radiosensitivity have different conclusions. But most experiments showed that SF2 is negatively related with cell radiosensitivity[6]. SF2 can be used as a marker for predicting the local control of head and neck tumor. It was reported that SF2 can predict the reaction after radiotherapy[7]. SF2 is related with the clinical prognosis of tumor[8]. In our study, SF2 was significantly related with the radiosensitivity of hepatic carcinoma cells, suggesting that SF2 can reflect the radiosensitivity of hepatic carcinoma cells.
In conclusion, SF2 is a reliable marker for predicting the radiosensitivity of hepatic carcinoma cells before and during the therapy.
Science Editor Wang XL Language Editor Elsevier HK
| 1. | Tamamoto T, Ohnishi K, Takahashi A, Wang X, Yosimura H, Ohishi H, Uchida H, Ohnishi T. Correlation between gamma-ray-induced G2 arrest and radioresistance in two human cancer cells. Int J Radiat Oncol Biol Phys. 1999;44:905-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Vral A, Thierens H, Baeyens A, De Ridder L. Chromosomal aberrations and in vitro radiosensitivity: intra-individual versus inter-individual variability. Toxicol Lett. 2004;149:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Bedford JS, Dewey WC. Radiation Research Society. 1952-2002. Historical and current highlights in radiation biology: has anything important been learned by irradiating cells? Radiat Res. 2002;158:251-291. [RCA] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | West CM, Davidson SE, Roberts SA, Hunter RD. Intrinsic radiosensitivity and prediction of patient response to radiotherapy for carcinoma of the cervix. Br J Cancer. 1993;68:819-823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 132] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | West CM, Davidson SE, Roberts SA, Hunter RD. The independence of intrinsic radiosensitivity as a prognostic factor for patient response to radiotherapy of carcinoma of the cervix. Br J Cancer. 1997;76:1184-1190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Björk-Eriksson T, West C, Karlsson E, Mercke C. Tumor radiosensitivity (SF2) is a prognostic factor for local control in head and neck cancers. Int J Radiat Oncol Biol Phys. 2000;46:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Britten RA, Evans AJ, Allalunis-Turner MJ, Franko AJ, Pearcey RG. Intratumoral heterogeneity as a confounding factor in clonogenic assays for tumour radioresponsiveness. Radiother Oncol. 1996;39:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Stausbøl-Grøn B, Overgaard J. Relationship between tumour cell in vitro radiosensitivity and clinical outcome after curative radiotherapy for squamous cell carcinoma of the head and neck. Radiother Oncol. 1999;50:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |