Published online Nov 7, 2005. doi: 10.3748/wjg.v11.i41.6459
Revised: April 2, 2005
Accepted: April 4, 2005
Published online: November 7, 2005
AIM: To investigate the influence of neutrophil adhesion molecule blockade with monoclonal antibody (MoAb CD11b) and E. coli lipopolysaccharide (LPS) administration on experimental acute pancreatitis (AP).
METHODS: AP was induced by four ip injections of cerulein (Cn) at 1-h intervals. MoAb CD 11b and LPS were administered at the beginning of the experiment.
RESULTS: The neutrophil count and chemiluminescence were diminished at the beginning of AP. The oxidative stress parameters were found within the pancreatic gland. MoAb CD 11b used for AP resulted in a significant reduction of pancreatic infiltration and pancreatitis oxidative stress parameters. Serum interleukin-6 (IL-6) was not detected in AP animals, whereas high serum IL-6 concentration was noted only in animals receiving LPS.
CONCLUSION: Neutrophils are involved in pancreatic damage in the early stage of AP. Neutrophil infiltration reduction protects the pancreatic gland from destruction during AP. LPS does not change the early course of Cn pancreatitis in rats.
- Citation: Hać S, Dobosz M, Kaczor JJ, Rzepko R, Aleksandrowicz-Wrona E, Wajda Z, Śledziński Z, Krajewski J. Neutrophil engagement and septic challenge in acute experimental pancreatitis in rats. World J Gastroenterol 2005; 11(41): 6459-6465
- URL: https://www.wjgnet.com/1007-9327/full/v11/i41/6459.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i41.6459
Acute pancreatitis (AP) is an insidious disease. Almost 75% of patients suffer from the mild edematous form of AP. About 25% of patients develop the severe disease with a mortality rate of 30-60%. The reasons of two different courses still remain unclear. One of the discussed reasons of organ dysfunction complicating AP is excessive leukocyte activation. Activation of inflammatory mediators [interleukins, leukotrienes, TNF-α, PAF, and lipopolysaccharide 9LPS)] within the pancreas may result in excessive damage within the pancreas, peripancreatic tissue or even distant organs[1-3]. The suggestion of neutrophil deteriorating role in human AP has largely been suggested. LPS, presented in the Gram-negative bacterial wall, has been studied extensively as an inflammatory stimulating factor contributing to the pathophysiology of conversion from mild to severe form of AP[4-6]. The pathophysiology of inflammatory process in AP leads to the immunomodulation concept. The idea is one of the most promising treatment options in AP nowadays. The early polymorphonuclear (PMN) requirement is well documented in AP.
The aim of this study was to investigate the PMN activation in the early stage of experimental cerulein (Cn) AP and the influence of mAb against adhesion molecule CD 11b on inflammatory response of the disease. Besides, whether LPS aggravated the early experimental AP in rats was also examined. The hypothesis of early aggravation of AP by LPS challenge was investigated.
The study was performed on male Wistar rats (180-200 g) that were kept on standard rat chow and fasted overnight before the experiment, but with free access to water. AP was induced by four ip injections of Cn at a dose of 15 μg/kg (Sigma Chemical Co., St. Louis, USA) in 1 mL of saline at 1-h intervals. MoAb CD 11b (POLATOM Świerk, Poland) was given iv once at the beginning of the experiment at a dose of 1 mg/kg. LPS (Sigma Chemical Co., St. Louis, USA) was administered ip at a dose of 10 mg/kg, simultaneously with the first Cn injection. Control animals were injected intravenously with an adequate volume of 0.9% saline solution. Five and nine hours from the beginning of the experiment, rats were anesthetized with sodium pentobarbital (25 mg/kg ip) and thoracolaparotomy was performed. Blood was aspirated into the test tubes on 30 U/mL heparin from left ventricle, the pancreas was excised, and animals were killed by exsanguinations. Pancreas specimen was taken for light microscopy and for determination of malonyldialdehyde (MDA), total sulfhydryl groups (SH) and myeloperoxidase (MPO). The study protocol was approved by The Local Ethic Committee for the Use of Experimental Animals of Medical University (Protocol No. 22/71).
Animals were divided into seven groups: group 1 (n = 14) control, group 2 (n = 16) AP, group 3 (n = 11) AP+MoAb CD 11b, group 4 (n = 11) AP+LPS, group 5 (n = 8) AP+MoAb CD 11b+LPS, group 6 (n = 8) MoAb CD 11b, group 7 (n = 11) LPS.
CL assay was performed in 1:200 diluted heparinized whole blood (30 IU/mL) to reduce artefacts. Diluent was prepared with PBS mixed with calcium and magnesium chloride (Biomed, Poland) and glucose (0.1%). Granulocyte CL was started by the phorbol-12-myristate-13-acetate (PMA, ICN Biomedicals Inc., USA). PMA (1 mg) was diluted with 1 mL dimethyl-sulfoxide (DMSO, Sigma) to the final concentration of 1.6×10-3 mol/L. PMA solution was diluted with PBS to the final concentration of 1.6×10-6 mol/L just before the measurement. Luminol solution was prepared with 1.77 mg dry substance (ICN Biomedicals Inc., USA) combined with 1 mL 0.4% NaOH and filled to 3 mL with PBS to obtain 3×10-5 mol/L concentration.
Transparent tubes containing 100 µL heparinized whole blood diluted 1:20 with PBS, 200 µL luminol (3×10-5 mol/L), 500 µL PBS and 200 µL PMA (1.6×10-5 mol/L) were analyzed in a FB 12 luminometer (Berthold Detection Systems, Germany) at 460-nm light length. The measurement was carried out at 10-s intervals for 20 min. All reagents were kept at 22°C during the procedure. The CL intensity was calculated in relative light units (RLU) per 2 000 of whole blood PMN cells, per minute (RLU/2 000 PMN ×min).
The measurement was carried out on the basis of commercial ELISA method (PMN-E, K6840 Immundiagnostic GmbH, Germany). PMN-E serum concentration was expressed as nanogram per milliliter.
A part of the pancreatic gland (at least 0.3 g wet mass) was taken during the laparotomy. The tissue was homogenized (Porter-Elvenheim ) immediately at 4 °C with PBS (pH 7.4). The volume of PBS was adequate to obtain 5% homogenate solution. Then the sample was centrifuged at 2 400 r/min for 10 min at 4 °C. The supernatant was taken for MPO, SH and MDA measurements. Two milliliters of the supernatant was mixed with 0.5 mL of 0.1% phenyl-methyl-sulfo-fluoride (Sigma Chemicals Co.) and 0.5 mL of 0.5% cetrimide (Merck). The mixture was centrifuged again at 2 400 r/min at 4 °C. MPO was measured using the ELISA method (Immundiagnostic GmbH, Germany). Pancreatic MPO concentration was expressed as nanogram per one gram of protein.
A total of 300 µL of pancreatic homogenate was diluted with sodium phosphate buffer (10 mmol/L) pH 8.0 to obtain 2.5% proteins concentration. Then 300 µL of sodium dodecyl sulfate (10%) was added to the sample to dissolve proteins in order to expose hidden sulfhydryls. Two thousand and four-hundred-microliter PBS was added, mixed and the absorbance was measured at 412 nm (Cecil Super Aquarius CE 9200). Then 300 µL of DTNB was added into the extract or 300 µL of PBS as blank to the reference mixture. Samples were incubated at 37°C in a water bath for 1 h. The absorbance was measured again. The concentration of SH groups was expressed as nanomole per milliliter.
MDA content was measured by spectrophotometer (LPO-586 Test Kit) assay (OxisResearch, Portland, USA), 600 µL of supernatant was mixed with 1 950 µL of R1 (10.3 nmol/L N-methyl-2-phenylindole solved in acetonitrile) and 450 µL 37% HCl. Samples were incubated in a water bath at 45 °C for 1 h, and then centrifuged at 3 500 r/min for 10 min. The absorbance of the supernatant was measured at 586 nm (Cecil Super Aquarius CE 9200).
The activity of α-amylase was measured using commercial kit RTU 63116 (BioMerieux). Lipase activity was determined using commercial kit Lipase Color 63109 (BioMerieux) in units per liter.
Hematoxylin-eosin standard staining was performed. An experienced pathologist performed the light microscopic examination. All samples were estimated with 200× magnification. Edema, vacuolization and necrosis of pancreatic cells were established in modified Spormann scale (0-3 points each parameter). PMN infiltration within the pancreatic parenchyma was calculated as mean number of neutrophils in 10 consecutive microscopic fields by a “blinded” pathologist.
Whole WBC and neutrophils were counted in Bürker’s camera following 1∶200 dilution with Türk’s solution. Cells were counted twice by experienced laboratory technician and the mean value was considered as the result.
Serum IL-6 concentration was determined using the commercial ELISA kit Rat-IL-6 (Biosource). The reaction was based on the peroxidation of tetra-methyl-benzydine. The absorbance measurement was made using an automatic reader (Organon Teknika; Reader 200) at 450-nm wave length.
Data were expressed as mean±SD. One-way analysis of variance with the post hoc analysis was employed to compare the groups. P<0.05 was considered statistically significant.
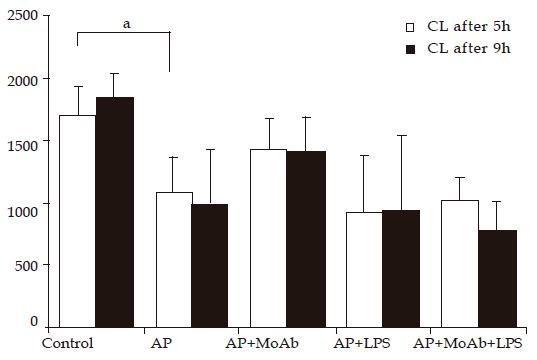
CL intensity of peripheral blood neutrophils was significantly lower in Cn AP group after 5 and 9 h of observation than that in control animals. CL was reduced from 1 700 to 1 086 RLU/min ×2 000 PMN in AP group, and from 1 842 to 988 RLU/min ×2 000 PMN after 9 h. MoAb CD 11b given before AP induction did not significantly decrease CL intensity in group 3, but the CL intensity decreased from 1 086 to 1 421 after 5 h and from 988 to 1 408 RLU/2 000 PMN ×min after 9 h of AP induction. No important reduction of CL was observed in AP groups receiving LPS or LPS and MoAb CD 11b. MoAb CD 11b given to healthy animals did not change CL value significantly. LPS given to healthy animals significantly reduced CL from 1 842 to 976 RLU/2 000 PMN ×min after 9 h of experiment (Figure 1).
Induction of Cn AP resulted in a significant reduction of peripheral WBC from 5 120 in control group to 2 204 cells/µL in AP group after 5 h of observation. PMN count in peripheral blood reduced from 1 230 to 614 cells/µL during the same period of time. MoAb CD 11b given before AP induction significantly increase WBC/PMN count in peripheral blood (from 2 204/614 to 3 942/2315 after 5 h of observation and from 4 225/1 286 to 4 037/1 120 cells/µL after 9 h of observation). LPS in AP group diminished peripheral WBC count 5 h after AP, while ameliorated their count 9 h after AP induction. LPS given with MoAb CD 11b in AP resulted in a time-dependent decrease of WBC count. MoAb CD 11b given to healthy animals did not change WBC count significantly. LPS given to healthy animals gradually reduced WBC count (Table 1).
| Group | WBC after 5 h | WBC after 9 h | PMN after 5 h | PMN after 9 h |
| (/µL) | (/µL) | (/µL) | (/µL) | |
| Control | 5 120 ± 1 220 | 5 597 ± 1 447 | 1230 ± 490 | 1 434 ± 569 |
| AP | 2 204a± 705a | 4 225 ±583 | 614a ± 229a | 1 286 ± 598 |
| AP+MoAb | 3 942c± 493c | 4 037 ±1 778 | 2 315c±850c | 1 120 ± 705 |
| AP+LPS | 1 678 ± 259 | 6 762c±580c | 1 047 ± 560 | 5 146c±1 124c |
| AP+MoAb | 4 087 ± 259 | 5 450 ± 580 | 1 830 ± 560 | 3 250 ± 1 124 |
| +LPS | ||||
| MoAb | 6 587±1 003 | 3 887 ± 1 612 | 2 200 ± 502 | 990 ± 307 |
| LPS | 3 771a±1 510a | 4 100 ± 1 824 | 2 892a±1 372a | 2 959a±1 553a |
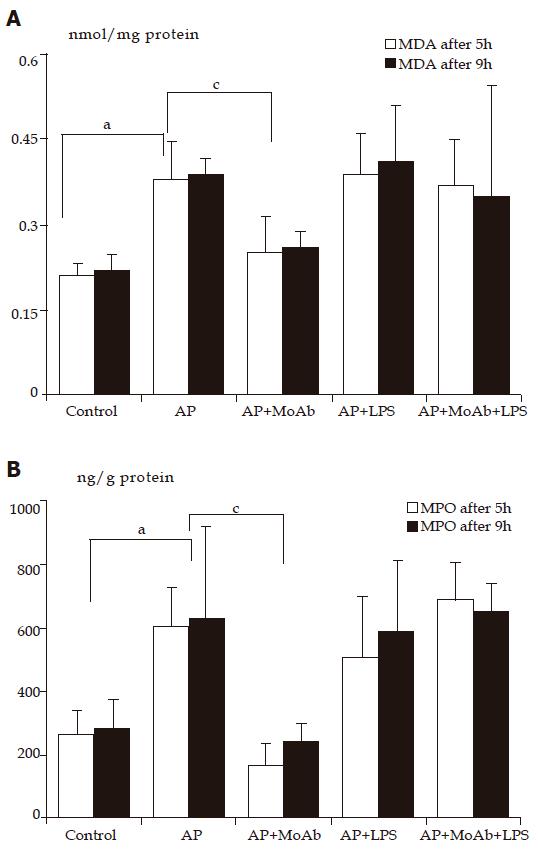
Sulfhydryl (-SH) compounds and malonyldialdehyde (MDA) concentration in pancreatic homogenate The concentration of (-SH) groups in pancreatic homogenate reduced from 60.3 nmol/mg protein in control group to 40.2 nmol/mg protein in AP group after 5 h of observation. Depletion of (-SH) compounds accompanied the elevation of MDA concentration in pancreatic homogenate from 0.21 nmol/mg protein in control group to 0.38 nmol/mg protein in AP group in the same period of time. After 9 h of observation concentration of (-SH) groups reduced from 64.7 nmol/mg in control group to 43.5 nmol/mg in AP group. LPS and MoAb CD 11b given together or separately in AP did not change the (-SH) group concentration in both periods of observation in comparison to AP group (group 2). MDA concentration in AP group treated with MoAb CD 11b reduced significantly from 0.38 to 0.25 after 5 h of observation and from 0.39 to 0.26 nmol/mg protein after 9 h of observation (Figure 2A and Table 2).
| Group | SH after 5 h | H after 9 h | MDA after 5 h | MDA after 9 h |
| Control | 60.3 ± 7.3 | 64.7 ± 8.3 | 0.21 ± 0.02 | 0.22 ± 0.03 |
| AP | 40.2a ± 8.5a | 43.5a ± 4.3a | 0.38a ± 0.07a | 0.39a ± 0.03a |
| AP+MoAb | 40.0 ± 6.5 | 46.7 ± 1.4 | 0.25c ± 0.06c | 0.26c ± 0.03c |
| AP+LPS | 40.0±6.8 | 41.1 ± 8.2 | 0.39 ± 0.07 | 0.41 ± 0.1 |
| AP+MoAb | 39.0 ± 6.7 | 40.4 ± 5.0 | 0.37 ± 0.08 | 0.35 ± 0.2 |
| +LPS | ||||
| MoAb | 63.4 ± 8.6 | 59.4 ± 9.0 | 0.2 ± 0.03 | 0.21 ± 0.04 |
| LPS | 52.0 ± 9.1 | 48.6 ± 4.7 | 0.28 ± 0.04 | 0.35 ± 0.05 |
AP significantly increased pancreatic MPO concentration from 260 in control animals to 602 ng/g protein in AP group after 5 h of observatiozn and from 280 ng/g protein to 626 ng/g protein after 9 h of observation compared to control group. Adhesion molecule blockade with MoAb CD 11b, prior to AP induction, protected the pancreatic gland from MPO elevation after 5 h (from 602 to 168 ng/g protein) and 9 h of observation (626-246 ng/g protein, Figure 2B). LPS or LPS with MoAb CD 11b given to AP groups did not change the course of pancreatic MPO activity (data not shown).
Cn (15 μg/kg for 4 h) caused transient hyperamylasemia and hyperlipasemia in all AP groups after 5 h of observation (groups 2-5). Animals receiving MoAb CD 11b alone or with LPS in AP (groups 3 and 5) had significantly lower α-amylase and lipase activity after 9 h of experiment compared to other AP groups (groups 2 and 4, Table 3).
| Group | Α-Amylase | Α-Amylase | Lipase | Lipase |
| after 5 h | after 9 h | after 5 h | after 9 h | |
| Control | 690 ± 120 | 743 ± 156 | 6 ± 8 | 8 ± 9 |
| AP | 8 286a ± 143a | 5 908a ± 640a | 2 140a ± 1 123a | 929a ± 252a |
| AP+MoAb | 5 119 ± 2 487 | 2 293c ± 1 739c | 1 704 ± 731 | 342c ± 178c |
| AP+LPS | 9 310 ± 7 552 | 4 001 ± 1 638 | 2 573 ± 2 133 | 1 257 ± 164 |
| AP+MoAb | 12 699c ± 4 978c | 2 719c ± 634c | 3 470 ± 1 832 | 305c ± 65c |
| +LPS | ||||
| MoAb | 696 ± 151 | 582 ± 273 | 21 ± 12 | 8 ± 4 |
| LPS | 584 ± 182 | 332 ± 93 | 11 ± 8 | 8 ± 4 |
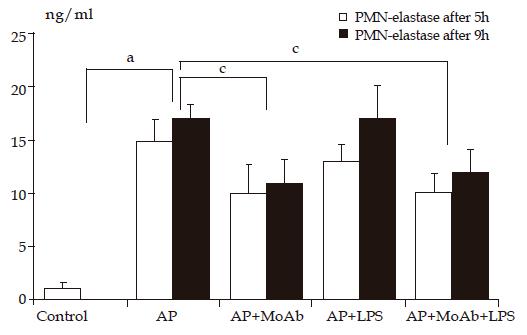
Cn-induced AP caused an elevation of plasma PMN-E in all AP groups (groups 2-5). AP animals receiving MoAb CD 11b alone or with LPS had significantly lower PMN-E activity in both periods compared to other AP groups. LPS given to healthy animals elevated plasma PMN-E in a time-dependent manner (3 ng/mL after 5 h and 8 ng/mL after 9 h of observation, Figure 3).
IL-6 was detectable in high concentration only after 5 h of observation in groups 4, 5, and 7 receiving LPS. AP did not correlate with IL-6 plasma level (Table 4).
Cn-induced AP caused marked pancreatic interstitial edema and acinar cell vacuolization, neutrophil infiltration and foci of necrosis. mAb given in AP reduced significantly interstitial neutrophil infiltration within the pancreas after 5 h of observation. LPS alone given to healthy animals resulted in a significant acceleration of pancreatic infiltration in comparison to control animals. Lipopolysaccharide given in AP did not result in significant exacerbation of microscopic findings within the pancreatic gland (Table 5).
| Houvs | Edema | Vacuolization | Infiltration | Necrosis | ||||
| 5 | 9 | 5 | 9 | 5 | 9 | 5 | 9 | |
| Group 1 | 0.00 | 0.00 | 0.00 | 0.00 | 1.1 | 1.21 | 0.00 | 0.00 |
| Control | ±0.00 | ±0.00 | ±0.00 | ±0.00 | ±0.9 | ±1.03 | ±0.00 | ±0.00 |
| Group 2 | 1.91a | 0.50 | 2.50a | 1.00 | 7.40a | 14.60a | 0.17 | 0.25 |
| AP | ±0.90a | ±0.57 | ±0.90a | ±1.41 | ±5.29a | ±2.37a | ±0.38 | ±0.50 |
| Group 3 | 2.28 | 1.50 | 1.71 | 1.50 | 3.87c | 10.95 | 0.00 | 0.00 |
| AP+MoAb CD11b | ±0.75 | ±1.00 | ±0.4 8 | ±1.00 | ±1.69c | ±0.92 | ±0.00 | ±0.00 |
| Group 4 | 1.28 | 2.00 | 1.57 | 1.25 | 10.94 | 17.92 | 0.14 | 0.00 |
| AP+LPS | ±1.25 | ±1.15 | ±1.39 | ±1.25 | ±3.02 | ±5.98 | ±0.37 | ±0.00 |
| Group 5 | 2.50 | 1.00 | 2.00 | 1.00 | 10.40 | 15.10 | 0.00 | 0.00 |
| AP+MoAbCD11b +LPS | ±1.00 | ±0.00 | ±1.15 | ±0.00 | ±8.30 | ±6.46 | ±0.00 | ±0.00 |
| Group 6 | 0.25 | 0.00 | 0.00 | 0.00 | 0.80 | 0.25 | 0.00 | 0.00 |
| Control+MoAb CD11b | ±0.50 | ±0.00 | ±0.00 | ±0.00 | ±1.04 | ±0.30 | ±0.00 | ±0.00 |
| Group 7 | 0.00 | 0.00 | 0.00 | 0.00 | 2.45 | 6.00a | 0.00 | 0.00 |
| Control+LPS | ±0.00 | ±0.00 | ±0.00 | ±0.00 | ±2.75 | ±4.11a | ±0.00 | ±0.00 |
Cn-induced AP is a widely accepted model of acute edematous pancreatitis in rats. The intraperitoneal route of four doses of Cn (15 µg/kg) at 1-h intervals is as effective as the intravenous route[7-9]. Based on the morphologic criteria of acute edematous pancreatitis including acinar cell vacuolization, interlobar and interstitial edema, leukocyte infiltration and foci of acinar cell necrosis, all animals receiving Cn revealed a remarkable hyperamylasemia and hyperlipasemia at the presented study.
The time of observation was established on the basis of preliminary experiments, maximum effect of Cn and T1/2 elimination of MoAb CD11b. The production of highly reactive molecules in PMN, as a result of respiratory burst activation, is an essential step in host defense against micro-organisms. The production of these highly reactive molecules can be measured by CL. CL is the process of light emission derived from the chemical reaction in which excited molecules decay the electronic basic state and emit photons. The CL intensity is directly proportional to cell activation and competence. Measurement of CL is a valuable and simple tool in studying PMN activity[10,11]. CL method is used in experimental and clinical medicine to evaluate the function of different cell types. CL detection is used as a label which allows signal amplification due to the enzyme-catalyzed reaction, thus obtaining a higher sensitivity[11]. The whole blood CL assay requires small blood samples and avoids artefacts due to cell isolation. The disadvantage of whole blood CL measurement is light extinguished by erythrocytes. Thus, highly diluted (final sample dilution 1:200) samples of whole blood are used. The diluted whole blood CL corresponds to individual neutrophil activity, because in this case interaction between cells and plasma is minimal[12].
Neutrophil infiltration is the universal feature of acute and chronic inflammatory process. The adhesion to vessel epithelium is followed by interstitial migration and action in the inflammatory focus[13-15]. This phenomenon is well documented. Neutrophil infiltration is the evidence of cell activation[16-20]. Experimental pancreatitis in the present study resulted in early PMN infiltration within the pancreatic gland. Whole blood white cells and neutrophils, in pancreatitis groups, reduced compared to control animals and also whole blood neutrophil CL reduced in AP groups during the same period of time. The phenomenon of low CL activity in pancreatitis group may be due to the migration of activated cells into interstitial space. After 9 h of experiment, the WBC and PMN count of AP group were similar to control one, because peripheral white cells perhaps were supplemented by immature cells from the bone marrow. However, the CL was still significantly lower, parallel to the results obtained in preliminary investigation[21]. The other possible explanation may be the action of plasma anti-inflammatory agents (cytokines: IL-10, IL-11; soluble receptors: IL-1ra, p75 or prostanoids PGE2 and PGI)[22].
Some data have confirmed that CL intensity is elevated in AP[23,24]. A different methodology of measurement may be the reason. The majority of investigators have analyzed the isolated neutrophils, whereas any isolation technique can change the neutrophil activity status[15,25]. On the other hand, the CL elevation may occur due to infection complicating AP clinical course.
CD11b is a well-known surface adhesion molecule of neutrophils. The use of mAb against CD11b results in competitive binding of specific adhesion molecules. Administration of MoAb CD11b in the present study significantly reduced the neutrophil infiltration within the pancreatic gland in AP animals. The pancreatic MPO tissue level was correlated to pancreatic neutrophil infiltration. Neutrophil depletion may protect pancreas from damage during AP as was observed by several other authors[16-18]. PAF antagonists reduce adhesion molecule expression and may prevent PMN infiltration of the pancreatic gland during AP. Another known pathomechanism of pancreatic damage during AP is oxidative stress. Several other authors documented that free radicals are generated in experimental AP[17-20]. Oxidative stress and lipid peroxidation debris elevated concentration was observed in animals with AP. MoAb CD11b administration during AP in the present study did not change the (-SH) groups concentration within the pancreatic gland, but the significant reduction of pancreatic MDA concentration was observed. The similar result was noticed in preliminary study group[21].
PMNs are the major source of oxygen radicals. Neutrophil NADPH-oxidase activation leads to the production of superoxide radicals during AP. Thus, MoAb 11b protects lipid peroxidation within pancreatic parenchyma in group 3. Our observations suggest that MoAb 11b induces neutrophil depletion in AP[16-20].
Substances which are thought to be effective in AP treatment (i.e., procaine, dextran, NO or heparin) reduce the aggregation of blood cells[14,26-29]. The role of ischemia-reperfusion and xanthine oxidase reaction is also the source of ROS in AP[26,29,30]. The aggregation of neutrophils and platelets may result in vessel obstruction. Kusterer et al[14] found that leukocyte adherence can be observed within the first 30 min from AP induction. The early neutrophil requirement determines the early adhesion molecule blockade necessity.
In our investigation, the IL-6 plasma concentration in AP groups was not elevated in both observation periods in comparison to control animals. IL-6 is a marker cytokine in severe human AP. Edematous AP may not demonstrate cytokinemia during the early stage of the disease[31-34]. On the other hand, some data suggest that IL-6 and other pro-inflammatory cytokines are elevated in Cn-induced AP[8,35]. The difference may be related to the different method of measurement. The biological method is not so specific compared to ELISA technique used in the present experiment[34]. On the other hand, IL-6 may be the marker of complications or SIRS but not AP “per se”. Numerous data have confirmed the early inflammatory process in the pancreatic parenchyma in early AP. Local cytokine concentration is much higher than the peripheral one[33,36]. The liver plays a cardinal role in cytokine clearance in the early stage of the disease. These facts confirm the local characteristic of AP in the edematous form of the disease.
The pathogenesis of AP is the involvement of bacterial LPS. LPS is the universal stimulator of inflammatory process. Digestive tract bacteria are the major source of LPS in AP. Mucosal damage is the reason of LPS detection in early course of AP. The infected pancreatic necrosis foci are responsible for LPS detection in late stage of AP. In the present work, group 7 was the evidence of LPS action on healthy animals. The LPS administered intraperitoneally worked as a systemic inflammatory agent. There was the elevation in peripheral PMN count, pancreatic infiltration and high plasma IL-6 concentration. AP rats receiving LPS (group 4) revealed slight depletion in circulating neutrophils compared to AP group after 5 h of experiment. It could be the consequence of larger neutrophil sequestration. After 9 h of experiment, elevated peripheral blood PMN count was elevated, but the CL intensity was as low as that in AP group. We observed elevation of circulating PMN count in group 4 after 9 h of observation. But CL intensity was not elevated in the same period. The difference in PMN count after 5 and 9 h of experiment might be the result of bone marrow or spleen release. Edematous AP seems to be the local inflammatory process, whereas LPS administration resulted in huge WBC and PMN count elevation after 9 h of observation.
The data confirm the early neutrophil requirement in AP. But surprisingly, the activation of circulating PMN was lower in AP than in control animals. This phenomenon may result from activated cell sequestration within the inflamed pancreatic gland. The adhesion molecule CD11b blockade and neutrophil infiltration reduction can protect the pancreatic parenchyma from damage. The early PMN involvement of PMN makes it difficult to conduct comparable clinical trials. Most AP patients admitted to the hospital have at least few hours of history. The idea of immunomodulation seems to be attractive but needs further investigations.
In conclusion, the circulating neutrophil and activity are reduced in the early period of Cn-induced AP in rats. Adhesion molecule CD11b blockade can prevent pancreatic infiltration and oxidative stress within the pancreatic gland during AP in rats. Lipopolysaccharide septic challenge does not change the early course of Cn-induced AP in rats.
The authors thank Mr. Anselm Berthold from Berthold Detection Systems for professional assistance and help, and Miss Justyna Hirsz for her language assistance.
Edited by Guo SY Language Editor Elsevier HK
| 1. | Johnson CD, Kingsnorth AN, Imrie CW, McMahon MJ, Neoptolemos JP, McKay C, Toh SK, Skaife P, Leeder PC, Wilson P. Double blind, randomised, placebo controlled study of a platelet activating factor antagonist, lexipafant, in the treatment and prevention of organ failure in predicted severe acute pancreatitis. Gut. 2001;48:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 231] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 2. | Kald B, Kald A, Ihse I, Tagesson C. Release of platelet-activating factor in acute experimental pancreatitis. Pancreas. 1993;8:440-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Yamano M, Umeda M, Miyata K, Yamada T. Protective effects of a PAF receptor antagonist and a neutrophil elastase inhibitor on multiple organ failure induced by cerulein plus lipopolysaccharide in rats. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:253-263. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Soong CV, Lewis HG, Halliday MI, Rowlands BJ. Intramucosal acidosis and the inflammatory response in acute pancreatitis. Am J Gastroenterol. 1999;94:2423-2429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Kimura Y, Hirota M, Okabe A, Inoue K, Kuwata K, Ohmuraya M, Ogawa M. Dynamic aspects of granulocyte activation in rat severe acute pancreatitis. Pancreas. 2003;27:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Gray KD, Simovic MO, Chapman WC, Blackwell TS, Christman JW, May AK, Parman KS, Stain SC. Endotoxin potentiates lung injury in cerulein-induced pancreatitis. Am J Surg. 2003;186:526-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Frossard JL, Saluja A, Bhagat L, Lee HS, Bhatia M, Hofbauer B, Steer ML. The role of intercellular adhesion molecule 1 and neutrophils in acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 1999;116:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 200] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Norman J, Franz M, Messina J, Riker A, Fabri PJ, Rosemurgy AS, Gower WR. Interleukin-1 receptor antagonist decreases severity of experimental acute pancreatitis. Surgery. 1995;117:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 163] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Vaccaro MI, Ropolo A, Grasso D, Calvo EL, Ferreria M, Iovanna JL, Lanosa G. Pancreatic acinar cells submitted to stress activate TNF-alpha gene expression. Biochem Biophys Res Commun. 2000;268:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Fujisawa M, Kojima K, Beppu T, Futagawa S, Kuwahara K, Hiramatsu K. Early diagnosis of postoperative infection: assessment of whole blood chemiluminescence. Surg Today. 2000;30:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Roda A, Pasini P, Guardigli M, Baraldini M, Musiani M, Mirasoli M. Bio- and chemiluminescence in bioanalysis. Fresenius J Anal Chem. 2000;366:752-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Bochev BG, Magrisso MJ, Bochev PG, Markova VI, Alexandrova ML. Dependence of whole blood luminol chemiluminescence on PMNL and RBC count. J Biochem Biophys Methods. 1993;27:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Hartwig W, Jimenez RE, Fernandez-del Castillo C, Kelliher A, Jones R, Warshaw AL. Expression of the adhesion molecules Mac-1 and L-selectin on neutrophils in acute pancreatitis is protease- and complement-dependent. Ann Surg. 2001;233:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Kusterer K, Poschmann T, Friedemann A, Enghofer M, Zendler S, Usadel KH. Arterial constriction, ischemia-reperfusion, and leukocyte adherence in acute pancreatitis. Am J Physiol. 1993;265:G165-G171. [PubMed] |
| 15. | Larvin M, Alexander DJ, Switala SF, McMahon MJ. Impaired mononuclear phagocyte function in patients with severe acute pancreatitis: evidence from studies of plasma clearance of trypsin and monocyte phagocytosis. Dig Dis Sci. 1993;38:18-27. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Bhatia M, Brady M, Shokuhi S, Christmas S, Neoptolemos JP, Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000;190:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 17. | Dabrowski A, Chwiećko M. Oxygen radicals mediate depletion of pancreatic sulfhydryl compounds in rats with cerulein-induced acute pancreatitis. Digestion. 1990;47:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Panés J, Perry M, Granger DN. Leukocyte-endothelial cell adhesion: avenues for therapeutic intervention. Br J Pharmacol. 1999;126:537-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 187] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 19. | Schulz HU, Niederau C, Klonowski-Stumpe H, Halangk W, Luthen R, Lippert H. Oxidative stress in acute pancreatitis. Hepatogastroenterology. 1999;46:2736-2750. [PubMed] |
| 20. | Tailor A, Das AM, Getting SJ, Flower RJ, Perretti M. Subacute treatment of rats with dexamethasone reduces ICAM-1 levels on circulating monocytes. Biochem Biophys Res Commun. 1997;231:675-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Hać S, Dobosz M, Kaczor J, Rzepko R. Influence of molecule CD 11b blockade on the course of acute ceruleine pancreatitis in rats. Exp Mol Pathol. 2004;77:57-65. [PubMed] |
| 22. | McKay C, Imrie CW, Baxter JN. Mononuclear phagocyte activation and acute pancreatitis. Scand J Gastroenterol Suppl. 1996;219:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Tsai K, Wang SS, Chen TS, Kong CW, Chang FY, Lee SD, Lu FJ. Oxidative stress: an important phenomenon with pathogenetic significance in the progression of acute pancreatitis. Gut. 1998;42:850-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Widdison AL, Cunningham S. Immune function early in acute pancreatitis. Br J Surg. 1996;83:633-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Pascual C, Karzai W, Meier-Hellmann A, Bredle DL, Reinhart K. A controlled study of leukocyte activation in septic patients. Intensive Care Med. 1997;23:743-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Closa D, Sabater L, Fernández-Cruz L, Prats N, Gelpí E, Roselló-Catafau J. Activation of alveolar macrophages in lung injury associated with experimental acute pancreatitis is mediated by the liver. Ann Surg. 1999;229:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Dobosz M, Hac S, Mionskowska L, Dobrowolski S, Wajda Z. Microcirculatory disturbances of the pancreas in cerulein-induced acute pancreatitis in rats with reference to L-arginine, heparin, and procaine treatment. Pharmacol Res. 1997;36:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Menger MD, Bonkhoff H, Vollmar B. Ischemia-reperfusion-induced pancreatic microvascular injury. An intravital fluorescence microscopic study in rats. Dig Dis Sci. 1996;41:823-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Sendur R, Pawlik WW. [Vascular factors in the mechanism of acute pancreatitis]. Przegl Lek. 1996;53:41-45. [PubMed] |
| 30. | Dobosz M, Wajda Z, Hać S, Myśliwska J, Bryl E, Mionskowska L, Roszkiewicz A, Myśliwski A. Nitric oxide, heparin and procaine treatment in experimental ceruleine-induced acute pancreatitis in rats. Arch Immunol Ther Exp (Warsz). 1999;47:155-160. [PubMed] |
| 31. | Gross V, Leser HG, Heinisch A, Schölmerich J. Inflammatory mediators and cytokines--new aspects of the pathophysiology and assessment of severity of acute pancreatitis? Hepatogastroenterology. 1993;40:522-530. [PubMed] |
| 32. | Kikuchi Y, Shimosegawa T, Satoh A, Abe R, Abe T, Koizumi M, Toyota T. The role of nitric oxide in mouse cerulein-induced pancreatitis with and without lipopolysaccharide pretreatment. Pancreas. 1996;12:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Okabe A, Hirota M, Nozawa F, Shibata M, Nakano S, Ogawa M. Altered cytokine response in rat acute pancreatitis complicated with endotoxemia. Pancreas. 2001;22:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Viedma JA, Pérez-Mateo M, Domínguez JE, Carballo F. Role of interleukin-6 in acute pancreatitis. Comparison with C-reactive protein and phospholipase A. Gut. 1992;33:1264-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 114] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | de Dios I, Perez M, de La Mano A, Sevillano S, Orfao A, Ramudo L, Manso MA. Contribution of circulating leukocytes to cytokine production in pancreatic duct obstruction-induced acute pancreatitis in rats. Cytokine. 2002;20:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Bhatnagar A, Wig JD, Majumdar S. Expression of activation, adhesion molecules and intracellular cytokines in acute pancreatitis. Immunol Lett. 2001;77:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |