Published online Jan 28, 2005. doi: 10.3748/wjg.v11.i4.597
Revised: April 8, 2004
Accepted: May 15, 2004
Published online: January 28, 2005
AIM: Taraxacum officinale (TO) has been frequently used as a remedy for inflammatory diseases. The aim of this study was to investigate the effect of TO on cholecystokinin (CCK)-octapeptide-induced acute pancreatitis in rats.
METHODS: TO at 10 mg/kg was orally administered, followed by 75 μg/kg CCK octapeptide injected subcutaneously three times after 1, 3 and 5 h. This whole procedure was repeated for 5 d. We determined the pancreatic weight/body weight ratio, the levels of pancreatic HSP60 and HSP72, and the secretion of pro-inflammatory cytokines. Repeated CCK octapeptide treatment resulted in typical laboratory and morphological changes of experimentally-induced pancreatitis.
RESULTS: TO significantly decreased the pancreatic weight/body weight ratio in CCK octapeptide-induced acute pancreatitis. TO also increased the pancreatic levels of HSP60 and HSP72. Additionally, the secretion of IL-6 and TNF-α decreased in the animals treated with TO.
CONCLUSION: TO may have a protective effect against CCK octapeptide-induced acute pancreatitis.
-
Citation: Seo SW, Koo HN, An HJ, Kwon KB, Lim BC, Seo EA, Ryu DG, Moon G, Kim HY, Kim HM, Hong SH.
Taraxacum officinale protects against cholecystokinin-induced acute pancreatitis in rats. World J Gastroenterol 2005; 11(4): 597-599 - URL: https://www.wjgnet.com/1007-9327/full/v11/i4/597.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i4.597
Cholecystokinin (CCK)-octapeptide is known to exert trophic effects on the pancreas in several species[1-3]. But high doses of CCK octapeptide fail to promote pancreatic trophism; moreover, they can induce oedematous pancreatitis[4-6].
Cells could respond to heat shock or other stresses by rapid synthesis of heat shock proteins (HSPs)[7]. The induction of heat shock responses enhances the ability of the cells to overcome the effects of stresses[8]. HSPs have been classified into six families according to their molecular mass (e.g., HSP60 and HSP72). It was reported that the preinduction of HSP expression had a protective effect against cerulein-induced pancreatitis in rats or choline-deficient ethionine-supplemented diet model pancreatitis in mice[9-15].
Besides, with increasing neutrophil migration to the pancreas, a variety of inflammatory cytokines are released. These include interleukin (IL)-1, IL-6, IL-8, platelet activating factor, and tumor necrosis factor (TNF). There is considerable evidence that pro-inflammatory cytokines play a central role in acute pancreatitis and may mediate the systemic complications of acute pancreatitis[16]. TNF has been implicated as an agent leading to progression of diseases, and IL-6 and IL-8 as indicators of disease severity.
Taraxacum officinale (TO) has been used in herbal medicines for its choleretic, diuretic and anti-inflammatory properties[17]. The effects of TO on pancreas and acute pancreatitis have not yet been investigated.
The aim of the present study was to investigate the effects of TO on the severity of CCK octapeptide-induced edematous pancreatitis. Moreover, we investigated the effects of TO and CCK octapeptide on pancreatic HSP60 and HSP72 synthesis. Additionally, we wished to evaluate whether TO could block pro-inflammatory cytokine synthesis during CCK octapeptide-induced acute pancreatitis.
Male Wistar rats weighing 240-260 g were used. The animals were kept at a constant room temperature of 25 °C with a 12 h light-dark cycle, and allowed free access to water and standard laboratory chow. The rats were fasted for 16 h before the induction of acute pancreatitis. In each experimental group five rats were used.
Avidin-peroxidase and 2’-AZINO-bis (3-ethylbenzithiazoline-6-sulfonic acid) tablet substrate were purchased from Sigma (St. Louis, MO, USA). Anti-HSP60 and HSP72 antibodies were purchased from Stressgen (British Columbia, Canada). Anti-rat TNF-α, and IL-6 antibodies were purchased from R&D Systems (Minneapolis, MN, USA).
TO was prepared by decocting the dried prescription of herbs with boiling distilled water. The decoction time was about 3 h. This plant was obtained from Dae-Hak Oriental Pharmacy (Iksan, South Korea). Their voucher specimens were deposited at the Herbarium at the College of Oriental Medicne, Kyung-Hee University.
TO at 10 mg/kg was administered orally, followed by CCK octapeptide injected subcutaneously at 75 μg/kg three times after 1, 3, and 5 h. This whole procedure was repeated for 5 d (n = 5). Other rodents (n = 5) received physiological saline orally instead of TO, but otherwise the protocol was the same as in TO-treated group. The animals were sacrificed by exsanguinations through the abdominal aorta 12 h after the last CCK octapeptide injection. Rats were killed for HSP60 and HSP 72 determinations. The pancreas was quickly removed, cleaned from fat and lymph nodes, weighed, and frozen at -70 °C until use. Rats were treated in accordance with the current law and the NIH Guide for Care and Use of Laboratory Animals.
Western blot analysis of pancreatic HSP60 and HSP72 was performed for the cytosolic fraction of the pancreas homogenates. Thirty micrograms of protein were loaded per lane. Samples were electrophoresed on a 10% SDS-PAGE according to the method of Laemmli[18]. The gels were either stained with Coomassie brilliant blue (to demonstrate equal loading of proteins for Western blot analysis) or transferred to a nitrocellulose membrane for 2 h at 300 mA. Membranes were blocked in 5% non-fat dry milk for 1 h and incubated with anti-HSP60 and anti-HSP72 antibodies. After washing in PBS-Tween-20 three times, the blot was incubated with secondary antibody for 30 min and the antibody-specific proteins were visualized by the enhanced chemiluminesence detection system according to the recommended procedure (Amersham Corp. Newark, NJ).
This ratio was utilized to evaluate the degree of pancreatic edema.
ELISA for IL-6 and TNF-α was carried out in duplicate in 96-well plates (Nunc, Denmark) coated with each of 100 μL aliquots of anti-rat IL-6 and TNF-α monoclonal antibodies at 1.0 μg/mL in PBS at pH 7.4 and was incubated overnight at 4 °C. The plates were washed in PBS containing 0.05% Tween-20 (Sigma, St. Louis, MO, USA) and blocked with PBS containing 1% BSA, 5% sucrose and 0.05% NaN3 for 1 h. After additional washes, standards were added and incubated at 37 °C for 2 h. After 2-h incubation at 37 °C, the wells were washed and then each of 0.2 μg/mL of biotinylated anti-rat IL-6 and TNF-α were added and again incubated at 37 °C for 2 h. After the wells were washed, avidin-peroxidase was added and plates were incubated for 20 min at 37 °C. Wells were again washed and ABTS substrate was added. Color development was measured at 405 nm using an automated microplate ELISA reader. A standard curve was run on each assay plate using recombinant IL-6 and TNF-α in serial dilutions.
Results were expressed as mean±SE. Differences between the experimental groups were evaluated by using analysis of variance. P<0.05 was accepted as statistically significant.
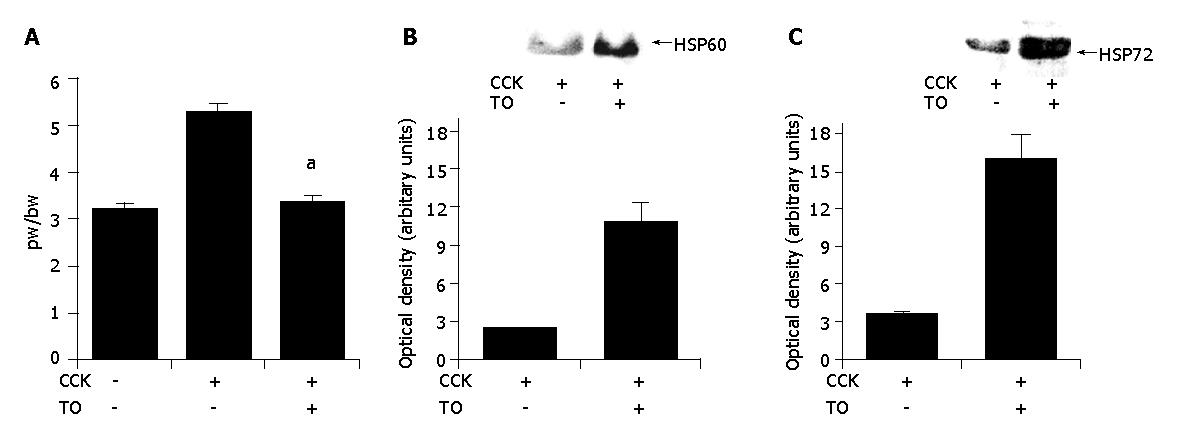
To assess the effect of TO on the pancreatic weight/body weight ratio, pancreatic weight was divided by the body weight of the rats. As shown in Figure 1A, in TO-treated group, pancreatic weight/body weight ratio (3.4±0.29) significantly decreased compared to the saline-treated group (5.3±0.38) (P<0.05).
Next, we studied the expression of HSP60 and HSP72 in pancreatitis. As shown in Figures 1B and 1C, in TO-treated group, the expression of pancreatic HSP60 and HSP72 was markedly increased in the animals with pancreatitis compared to the saline-treated group.
Injections of CCK octapeptide increased the pancreatic TNF-α and IL-6 levels over time. TO pretreatment significantly decreased the levels of IL-6 production (150±90 pg/mL) during CCK octapeptide-induced acute pancreatitis vs the saline-treated groups (440±30 pg/mL). Moreover, TO pretreatment decreased the levels of TNF-α production during CCK octapeptide-induced acute pancreatitis vs the saline-treated groups. However, the statistical difference was very weak (P = 0.072) (Table 1).
TO has long been used for medicinal purposes due to its choleretic, diuretic and anti-inflammatory activities[19]. Our study was designed to examine the in vivo dynamics of pancreatic HSP60 and HSP72 induction in response to CCK octapeptide or TO. HSPs could play a universal role in the maintenance of cellular homeostasis[20]. They were expressed constitutively and/or at elevated levels upon the exposure of cells to a variety of stress conditions in every organ, including the pancreas[9,21]. The HSPs have been found to be involved in the synthesis, degradation, folding, transport, and translocation of proteins[8,9]. Whereas many diseases could result in increased levels of HSPs, Strowski et al[22] demonstrated that cerulein-induced pancreatitis reduced the levels of pancreatic HSPs. This observation even suggests that the low levels of pancreatic HSPs might be involved in the development of CCK octapeptide-induced pancreatitis. Moreover, an increasing body of evidence from experimental animal studies has documented an essential role of HSPs in the prevention of acute pancreatitis. HSP preinduction is known to protect the pancreas from cerulein-induced pancreatitis in rats or choline-deficient ethionine-supplemented diet model pancreatitis in mice[11-17]. In accordance with Strowski et al, we showed that supramaximal doses of CCK octapeptide could reduce the levels of HSP60 and HSP72. However, this decrease was ameliorated by administration of TO.
IL-6, a principal mediator of acute phase response, is primarily released from activated mononuclear phagocytes. Pooran et al showed that IL-6 levels in severe pancreatitis compared with mild pancreatitis were significantly elevated[23,24]. TNF-α is a predominantly macrophage-derived cytokine. It is produced within the pancreas by leukocytes that could invade the parenchyma during acute pancreatitis[25,26]. It is also the primary stimulus of IL-6 and IL-8 production and is known to initiate and propagate almost all the detrimental consequences in severe sepsis[27]. We demonstrated that TO could reduce IL-6 and TNF-α production during CCK octapeptide-induced acute pancreatitis in rats.
In conclusion, TO pretreatment can ameliorate the severity of CCK octapeptide-induced pancreatitis in rats. TO can protect against CCK octapeptide-induced acute pancreatitis in rats. The beneficial nature of TO in this acute pancreatitis model warrants further investigation.
Co-correspondents: Hyung-Min Kim
Edited by Wang XL and Zhu LH
| 1. | Solomon TE. Regulation of pancreatic secretion. Clin Gastroenterol. 1984;13:657-678. [PubMed] |
| 2. | Glasbrenner B, Adler G. Pathophysiology of acute pancreatitis. Hepatogastroenterology. 1993;40:517-521. [PubMed] |
| 3. | Niederau C, Lüthen R, Heintges T. Effects of CCK on pancreatic function and morphology. Ann N Y Acad Sci. 1994;713:180-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Solcia E, Capella C, Riva C, Rindi G, Polak JM. The morphology and neuroendocrine profile of pancreatic epithelial VIPomas and extrapancreatic, VIP-producing, neurogenic tumors. Ann N Y Acad Sci. 1988;527:508-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977;373:97-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 408] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Hietaranta AJ, Aho HJ, Nevalainen TJ. Pancreatic phospholipase A2 in cerulein-induced acute pancreatitis in the rat. Int J Pancreatol. 1993;14:261-267. [PubMed] |
| 7. | Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3214] [Cited by in RCA: 3065] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 8. | Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72:1063-1081. [PubMed] |
| 9. | Otaka M, Itoh H, Kuwabara T, Zeniya A, Fujimori S, Otani S, Tashima Y, Masamune O. Induction of heat shock protein and prevention of caerulein-induced pancreatitis by water-immersion stress in rats. Int J Biochem. 1994;26:805-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Otaka M, Okuyama A, Otani S, Jin M, Itoh S, Itoh H, Iwabuchi A, Sasahara H, Odashima M, Tashima Y. Differential induction of HSP60 and HSP72 by different stress situations in rats. Correlation with cerulein-induced pancreatitis. Dig Dis Sci. 1997;42:1473-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Wagner AC, Weber H, Jonas L, Nizze H, Strowski M, Fiedler F, Printz H, Steffen H, Göke B. Hyperthermia induces heat shock protein expression and protection against cerulein-induced pancreatitis in rats. Gastroenterology. 1996;111:1333-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Grisé K, Kim F, McFadden D. Hyperthermia induces heat-shock protein expression, reduces pancreatic injury, and improves survival in necrotizing pancreatitis. Pancreas. 2000;21:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Lee HS, Bhagat L, Frossard JL, Hietaranta A, Singh VP, Steer ML, Saluja AK. Water immersion stress induces heat shock protein 60 expression and protects against pancreatitis in rats. Gastroenterology. 2000;119:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Rakonczay Z, Takács T, Mándi Y, Iványi B, Varga S, Pápai G, Boros I, Lonovics J. Water immersion pretreatment decreases pro-inflammatory cytokine production in cholecystokinin-octapeptide-induced acute pancreatitis in rats: possible role of HSP72. Int J Hyperthermia. 2001;17:520-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Frossard JL, Pastor CM, Hadengue A. Effect of hyperthermia on NF-kappaB binding activity in cerulein-induced acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1157-G1162. [PubMed] |
| 16. | Kusske AM, Rongione AJ, Reber HA. Cytokines and acute pancreatitis. Gastroenterology. 1996;110:639-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 132] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Kisiel W, Barszcz B. Further sesquiterpenoids and phenolics from Taraxacum officinale. Fitoterapia. 2000;71:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185380] [Cited by in RCA: 188820] [Article Influence: 3433.1] [Reference Citation Analysis (0)] |
| 19. | Koo HN, Hong SH, Song BK, Kim CH, Yoo YH, Kim HM. Taraxacum officinale induces cytotoxicity through TNF-alpha and IL-1alpha secretion in Hep G2 cells. Life Sci. 2004;74:1149-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Rakonczay Z, Iványi B, Varga I, Boros I, Jednákovits A, Németh I, Lonovics J, Takács T. Nontoxic heat shock protein coinducer BRX-220 protects against acute pancreatitis in rats. Free Radic Biol Med. 2002;32:1283-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Schäfer C, Williams JA. Stress kinases and heat shock proteins in the pancreas: possible roles in normal function and disease. J Gastroenterol. 2000;35:1-9. [PubMed] |
| 22. | Strowski MZ, Sparmann G, Weber H, Fiedler F, Printz H, Jonas L, Göke B, Wagner AC. Caerulein pancreatitis increases mRNA but reduces protein levels of rat pancreatic heat shock proteins. Am J Physiol. 1997;273:G937-G945. [PubMed] |
| 23. | Heath DI, Cruickshank A, Gudgeon M, Jehanli A, Shenkin A, Imrie CW. Role of interleukin-6 in mediating the acute phase protein response and potential as an early means of severity assessment in acute pancreatitis. Gut. 1993;34:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 212] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Pooran N, Indaram A, Singh P, Bank S. Cytokines (IL-6, IL-8, TNF): early and reliable predictors of severe acute pancreatitis. J Clin Gastroenterol. 2003;37:263-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Norman JG, Fink GW, Denham W, Yang J, Carter G, Sexton C, Falkner J, Gower WR, Franz MG. Tissue-specific cytokine production during experimental acute pancreatitis. A probable mechanism for distant organ dysfunction. Dig Dis Sci. 1997;42:1783-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 174] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 26. | Norman JG, Fink GW, Franz MG. Acute pancreatitis induces intrapancreatic tumor necrosis factor gene expression. Arch Surg. 1995;130:966-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 149] [Article Influence: 5.0] [Reference Citation Analysis (0)] |