Published online Jan 28, 2005. doi: 10.3748/wjg.v11.i4.577
Revised: May 14, 2004
Accepted: June 18, 2004
Published online: January 28, 2005
AIM: Although polysaccharides from Phellinus mushrooms are a well-known material with anti-tumor properties, there is no information about the effect of polysaccharides from Phellinus gilvus (PG) on tumor. The modulating effect of polysaccharides isolated from PG on the benzo(a)pyrene (BaP)-induced forestomach carcinogenesis in ICR female mice was investigated in this study.
METHODS: A forestomach carcinogenesis model was established in 40 ICR female mice receiving oral administration of BaP for 4 wk. The mice were randomly assigned to 4 groups (10 each). The mice in each group were treated with sterile water or PG for 4 and 8 wk (SW4, PGW4, SW8, and PGW8 groups). Eight or 12 wk after the first dose of BaP, forestomachs were removed for histopathological and RT-PCR analysis.
RESULTS: In histopathological changes and RT-PCR analysis, sterile water-treated mice showed significant hyperplasia of the gastric mucosa with a significantly increased expression of mutant p53 mRNA compared to mice treated with PG for 8 wk.
CONCLUSION: Polysaccharides isolated from PG may inhibit BaP-induced forestomach carcinogenesis in mice bydown-regulating mutant p53 expression.
-
Citation: Bae JS, Jang KH, Yim H, Park SC, Jin HK. Inhibitory effects of polysaccharides isolated from
Phellinus gilvus on benzo(a)pyrene-induced forestomach carcinogenesis in mice. World J Gastroenterol 2005; 11(4): 577-579 - URL: https://www.wjgnet.com/1007-9327/full/v11/i4/577.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i4.577
Gastric tumor is the most frequent malignancy of the gastrointestinal tract in East Asian populations and the second most common cause of cancer-related death in the world. Its mortality associated with gastric tumor has remained relatively stable over the past 20 years, suggesting that new therapies to combat this problem are urgently needed[1].
The use of medicinal plants in modern medicine for the prevention or treatment of the tumor is an important aspect. In a recent study, several natural products have been investigated on inhibitory effect of gastric tumor using animal models[2,3]. Among these products, polysaccharides isolated from Phellinus mushrooms have received special attention due to their anti-tumor[4-6] and immunostimulating effects[7,8].
We have previously demonstrated that a kind of Phellinus mushrooms, PG, has various biological activities related to inflammation, including inhibition of pulmonary inflammation[9], prevention of intraperitoneal adhesion under infectious circumstances[10], and promotion of dermal wound healing (unpublished data). PG also has advantages over the other Phellinus mushrooms in that it has a very short growth period (3 mo) making it cheaper to produce. Thus, we predict that medical application of PG has advantages in medical cost-cutting, and will benefit to health in future. However, the role of polysaccharides isolated from PG in inhibiting tumors has not been demonstrated in vivo.
In this work, we investigated whether polysaccharides isolated from PG could protect mouse forestomach against the neoplastic effects of BaP using histopathological and RT-PCR analysis.
The fruiting body of PG was kindly provided by Gyeongbuk Agricultural Technology Administration (Daegu, Korea). A seed culture was grown in a 250 mL flask containing 50 mL of PMP medium (2.4% potato/dextrose broth plus 1% malt extract, 0.1% peptone) at 28 °C on a rotary incubator at 150 r/min for 4 d. To obtain fruiting bodies of PG, a culture was grown in an oak sawdust block for 90 d. The yield of fruiting bodies was 97 g dried weight per block. It was extracted by optimal conditions of water extraction for maximal anti-tumor activity, distilled water (1:25) at 100 °C for 10 h (unpublished data). The recovery procedure of polysaccharides from water extract of PG followed a previously established method[10,11]. The material was stored at 4 °C until used.
Fifty female ICR mice, 6- to 8-wk old, were purchased from Charles River Laboratory (Bio Genomics, Korea) and acclimatized under the controlled condition for 1 wk. All animals were maintained with commercial rat diet (Orient Inc., Korea) and water ad libitum. BaP-induced forestomach tumorigenesis in mice was induced according to the procedure described by Wattenberg[12]. The animals were administered with 100 μL corn oil (CO group) or 1 mg of BaP in 100 μL corn oil by gavage twice a week for 4 wk. After the last dose of BaP, the mice were randomly assigned to 4 groups (10 each). The mice in each group were treated with sterile water or PG for 4 and 8 wk, respectively (SW4, PGW4, SW8, and PGW8 groups). The animals of PG treated groups received 1 mg/mL of polysaccharides isolated from PG as a sole source of drinking water for 4 or 8 wk.
Eight or 12 wk after the first dose of BaP, half the animals in each group received a lethal injection of 2.5% avertin and were immediately perfused with ice-cold phosphate buffer (PB) followed by 4% paraformaldehyde in PB. The forestomachs were then removed carefully and fixed in 10% buffered formalin. After routine tissue processing, serial sections (5 μm) were stained with hematoxylin and eosin. The dynamic processes of carcinogenesis in each period were assessed by light microscopy.
The remaining animals in each group were sacrificed with carbon dioxide asphyxiation 8 or 12 wk after the first dose of BaP. Removed forestomachs were stored at -80 °C until further processing. Total cellular RNA was extracted from forestomachs using a monophasic solution of phenol and isothiocyanate (TRIzol Reagent, Gibco). A 1 μg total RNA was subjected to the first-strand cDNA synthesis in a TOUCHgene DNA thermal cycler (Techne (Cambridge) Limited, UK) at 42 °C for 60 min followed by enzyme denaturation at 94 °C for 2 min. All reagents were obtained from Promega (Madison, WI, USA). The expression of p53 was semiquantitatively detected and β-actin was used as an internal standard. Sequences of primers used for amplification were as follows: p53: sense, 5’-GGAGGTTGT GAGGCGCTGC-3’, antisense, 5’-CACGCACCTCAAAGC TGTTC-3’; β-actin: sense, 5’-AGCGGGAAATCGTGCGTGAC-3’, antisense, 5’-ACTCCTGCTTGCTGATCCACATC-3’. PCR was conducted in a TOUCHgene DNA thermal cycler. PCR products were separated by electrophoresis using 2% agarose gels stained with ethidium bromide to visualize cDNA products.
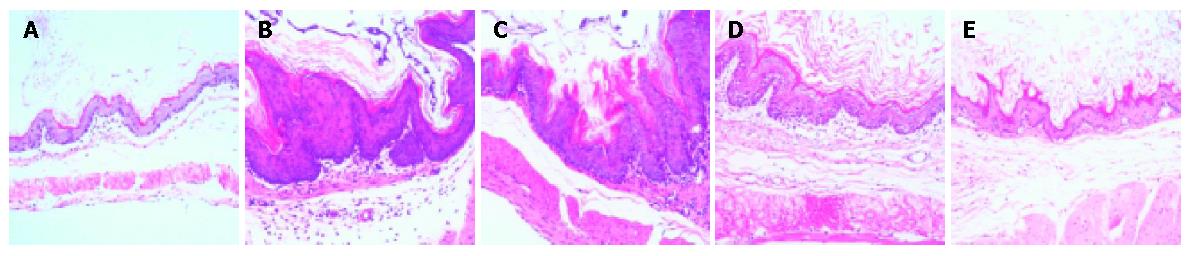
Histopathological changes in mice treated with sterile water for 4 and 8 wk after the last administration of BaP manifested as significant hyperplasia of the gastric mucosa, referred to as “precancerous lesions” with a few papillomas. The thickness of epidermis was not uniform. There was no significant difference between SW4 and SW8 groups.
Conversely, mice treated with PG for 4 wk showed only marginal hyperplasia with no papillomas. Mice treated with PG for 8 wk after the last administration of BaP had the same histopathological appearance as control mice treated with corn oil (Figure 1). This evidence supported the hypothesis that polysaccharides isolated from PG could inhibit BaP-induced forestomach carcinogenesis in mice. The dynamic processes of carcinogenesis in each period are shown in Table 1.
| Treatments | 4 wk | 8 wk | ||
| H (%) | P (%) | H (%) | P (%) | |
| SW | 81.5 | 3.7 | 97.1 | 5.8 |
| PG | 37.3 | 0 | 12.8 | 0 |
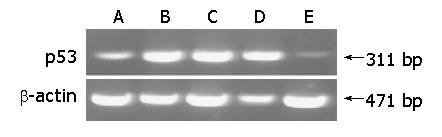
RT-PCR was carried out to demonstrate the effect of PG on the release of p53. In the SW groups treated with only BaP, p53 mRNA was highly expressed compared with that in the PGW8 and CO groups. β-actin transcript levels among all groups were the same (Figure 2).
The major aim of this study was to investigate whether polysaccharides isolated from PG could inhibit BaP-induced forestomach carcinogenesis in mice in order to assess the potential plants such as PG for future therapies.
There are approximately 220 known species of Phellinus mushrooms in the world, and they were found mainly in tropical areas of America and Africa[13]. Many kinds of Phellinus (e.g., P. linteus, P. baumii, P. igniarius, and P. pini, ets.) are known to have different medicinal effects[14-20]. Among them, P. linteus is well known as one of the most popular medicinal mushrooms due to its high anti-tumor[4] and immunostimulating[7,8,] activities. It has been utilized medicinally in Korea and Japan. Here we showed that polysaccharides isolated from a kind of Phellinus, PG, suppressed forestomach carcinogenesis in mice. This suggests a potential therapeutic role of PG as an adjuvant for the treatment of tumors.
In modern medicine, natural products like PG for the prevention or treatment of tumors are attractive chemopreventive agents because of their very low clinical toxicity compared with chemical anti-tumor drugs[21,22]. Recently, the safety of a single orally-administered dose of PG was demonstrated in our previous work[23]. In this study, we also observed no body weight change during PG treatment (data not shown). These results indicated that PG was a chemopreventive agent with a very low clinical toxicity.
Many oncogene and tumor suppressor gene products can regulate and execute apoptosis. Among them, the p53 tumor suppressor gene plays an important role in both apoptosis and DNA repair pathways. In various tumors, p53 is an essential gene for inducing apoptosis and the level is increased in anti-tumor therapy. However, when p53 also mutated in response to intracellular and extracellular stress signals, e.g., chemically induced DNA damage, the level was increased. Many studies have demonstrated that treatment with BaP seemed to increase p53 mRNA by inducing p53 mutation in some models[24,25]. In the present study, mice treated with sterile water after administration of BaP had higher levels of p53 mRNA expression compared with CO and PGW8 groups, suggesting that p53 mutated by BaP could decrease cancer cell apoptosis and provide selective growth superiority to cancer cells. Especially, in the PGW8 group, the expression of p53 mRNA was significantly lower than that in SW4 and SW8 groups. Thus, we can conclude that polysaccharides isolated from PG may induce cancer cell apoptosis by down-regulating mutant p53 mRNA expression.
Many investigators have been demonstrated that the mechanism of Phellinus mushroom anti-tumor action involves multiple processes[18,22,26,27]. In our models, we observed the expression of mutant p53 mRNA of PG. We are actively exploring the mechanism of angiogenesis or apoptosis in tumor due to PG-like inhibition.
In conclusion, polysaccharides isolated from PG may protect mouse forestomach against the neoplastic effects of BaP by rapid down-regulating of mutant p53 mRNA expression. PG is a pharmacologic agent that rapidly enhances host resistance to tumors. Further studies regarding its mechanism are needed to promote the clinical applications of PG in cancer therapy.
The authors thank J.K. Lee, S.I. Kim and H.B. Yoon for their technical assistance. Thanks are also due to Dr. J. Carter, Department of Psychiatry and Behavioural Sciences, University College, London for the preparation of this manuscript.
Co-correspondents: Hee-Kyung Jin and Kwang-Ho Jang
Edited by Wang XL
| 1. | Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80:827-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 2. | Wu K, Shan YJ, Zhao Y, Yu JW, Liu BH. Inhibitory effects of RRR-alpha-tocopheryl succinate on benzo(a)pyrene (B(a)P)-induced forestomach carcinogenesis in female mice. World J Gastroenterol. 2001;7:60-65. [PubMed] |
| 3. | Jagetia GC, Baliga MS, Venkatesh P. Effect of Sapthaparna (Alstonia scholaris Linn) in modulating the benzo(a)pyrene-induced forestomach carcinogenesis in mice. Toxicol Lett. 2003;144:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Han SB, Lee CW, Jeon YJ, Hong ND, Yoo ID, Yang KH, Kim HM. The inhibitory effect of polysaccharides isolated from Phellinus linteus on tumor growth and metastasis. Immunopharmacology. 1999;41:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 168] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Bae JS, Hwang MH, Jang KH, Rhee MH, Lee KW, Jo WS, Choi SG, Yun HI, Yim JH, Kim JC. Comparative antitumor activity of water extracts from fruiting body of Phellinus linteus, Phellinus baumii and Phellinus gilvus. J Toxicol Pub Health. 2004;20:37-42. |
| 6. | Ajith TA, Janardhanan KK. Cytotoxic and antitumor activities of a polypore macrofungus, Phellinus rimosus (Berk) Pilat. J Ethnopharmacol. 2003;84:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Lee JH, Cho SM, Song KS, Hong ND, Yoo ID. Characterization of carbohydrate-peptide linkage of acidic heteroglycopeptide with immuno-stimulating activity from mycelium of Phellinus linteus. Chem Pharm Bull (Tokyo). 1996;44:1093-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Kim HM, Han SB, Oh GT, Kim YH, Hong DH, Hong ND, Yoo ID. Stimulation of humoral and cell mediated immunity by polysaccharide from mushroom Phellinus linteus. Int J Immunopharmacol. 1996;18:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 172] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Jang BS, Kim JC, Bae JS, Rhee MH, Jang KH, Song JC, Kwon OD, Park SC. Extracts of Phellinus gilvus and Phellinus baumii inhibit pulmonary inflammation induced by lipopolysaccharide in rats. Biotechnol Lett. 2004;26:31-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Bae JS, Jin HK, Jang KH. The effect of polysaccharides and carboxymethylcellulose combination to prevent intraperitoneal adhesion and abscess formation in a rat peritonitis model. J Vet Med Sci. 2004;66:1205-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Kim DH, Yang BK, Jeong SC, Park JB, Cho SP, Das S, Yun JW, Song CH. Production of a hypoglycemic, extracellular polysaccharide from the submerged culture of the mushroom, Phellinus linteus. Biotechnol Lett. 2001;23:513-517. [DOI] [Full Text] |
| 12. | Wattenberg LW, Coccia JB, Lam LK. Inhibitory effects of phenolic compounds on benzo(a)pyrene-induced neoplasia. Cancer Res. 1980;40:2820-2823. [PubMed] |
| 13. | Kim GY, Park HS, Nam BH, Lee SJ, Lee JD. Purification and characterization of acidic proteo-heteroglycan from the fruiting body of Phellinus linteus (Berk. & amp; M.A. Curtis) Teng. Bioresour Technol. 2003;89:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Jung HG, Valdez FR, Abad AR, Blanchette RA, Hatfield RD. Effect of white rot basidiomycetes on chemical composition and in vitro digestibility of oat straw and alfalfa stems. J Anim Sci. 1992;70:1928-1935. [PubMed] |
| 15. | Cho JH, Cho SD, Hu H, Kim SH, Lee SK, Lee YS, Kang KS. The roles of ERK1/2 and p38 MAP kinases in the preventive mechanisms of mushroom Phellinus linteus against the inhibition of gap junctional intercellular communication by hydrogen peroxide. Carcinogenesis. 2002;23:1163-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Park SK, Kim GY, Lim JY, Kwak JY, Bae YS, Lee JD, Oh YH, Ahn SC, Park YM. Acidic polysaccharides isolated from Phellinus linteus induce phenotypic and functional maturation of murine dendritic cells. Biochem Biophys Res Commun. 2003;312:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Ajith TA, Janardhanan KK. Antioxidant and antihepatotoxic activities of Phellinus rimosus (Berk) Pilat. J Ethnopharmacol. 2002;81:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Jeon TI, Hwang SG, Lim BO, Park DK. Extracts of Phellinus linteus grown on germinated brown rice suppress liver damage induced by carbon tetrachloride in rats. Biotechnol Lett. 2003;25:2093-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Shon YH, Nam KS. Antimutagenicity and induction of anticarcinogenic phase II enzymes by basidiomycetes. J Ethnopharmacol. 2001;77:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Shon YH, Nam KS. Inhibition of cytochrome P450 isozymes and ornithine decarboxylase activities by polysaccharides from soybeans fermented with Phellinus igniarius or Agrocybe cylindracea. Biotechnol Lett. 2004;26:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Lee KH. Current developments in the discovery and design of new drug candidates from plant natural product leads. J Nat Prod. 2004;67:273-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Deshpande SS, Ingle AD, Maru GB. Inhibitory effects of curcumin-free aqueous turmeric extract on benzo[a]pyrene-induced forestomach papillomas in mice. Cancer Lett. 1997;118:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Bae JS, Jang KH, Choi SG, Jo WS, Rhee MH, Park SC. Acute oral toxicity of extract derived from fruiting body of Phellinus gilvus in rats. J Toxicol Pub Health. 2003;19:211-215. |
| 24. | Pei XH, Nakanishi Y, Takayama K, Bai F, Hara N. Benzo[a]pyrene activates the human p53 gene through induction of nuclear factor kappaB activity. J Biol Chem. 1999;274:35240-35246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Serpi R, Vähäkangas K. Benzo(a)pyrene-induced changes in p53 and related proteins in mouse skin. Pharmacol Toxicol. 2003;92:242-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Kim GY, Oh YH, Park YM. Acidic polysaccharide isolated from Phellinus linteus induces nitric oxide-mediated tumoricidal activity of macrophages through protein tyrosine kinase and protein kinase C. Biochem Biophys Res Commun. 2003;309:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Kim HM, Han SB, Oh GT, Kim YH, Hong DH, Hong ND, Yoo ID. Stimulation of humoral and cell mediated immunity by polysaccharide from mushroom Phellinus linteus. Int J Immunopharmacol. 1996;18:295-303. |