Published online Jan 28, 2005. doi: 10.3748/wjg.v11.i4.567
Revised: April 7, 2004
Accepted: May 29, 2004
Published online: January 28, 2005
AIM: To evaluate the role of intestinal endotoxemia in the genesis of hepatopulmonary syndrome.
METHODS: A rat model of cirrhosis was prepared with the method of compound factors. At the end of the eighth week, rats with cirrhosis were treated with 300 μg LPS/100 g body weight, and 1 g/rat of glycine about four h prior to LPS. After three h of LPS treatment, blood and tissues were collected for various measurements. Kupffer cells were isolated from male Wistar rats and cultured, and divided into five groups. Supernatant was harvested at 3 h after treatment with LPS for measurement of tumor necrosis factor-alpha (TNF-α).
RESULTS: Our results showed that in rats with cirrhosis, slowed and deepened breath with occasional pause was. PaO2, PaCO2 and standard bicarbonate (SB) in arterial blood were decreased. Arterial O2 and actual bicarbonate (AB) were markedly decreased. There was a close correlation between decreased O2 and endotoxin. Metabolic acidosis accompanying respiratory alkalosis was the primary type of acid-base imbalance. The alveolar-arterial oxygen gradient was sharply widened. Massive accumulation of giant macrophages in the alveolar spaces and its wall and widened alveolar wall architecture were observed. The number of bacterial translocations in mesenteric lymph nodes increased. The ratio of TC99M-MAA brain-over-lung radioactivity rose. Endotoxin, and TNF-α, endothelin-1 (ET-1), nitric oxide (NO) in plasma and ET-1, carbon monoxide (CO) in lung homogenates increased. After administration of a given dosage of LPS in rats with cirrhosis, various pathological parameters worsened. Plasma level of endotoxin was related to TNF-α, ET-1, NO in plasma and ET-1, NO, CO in lung homogenates. TNF-α level was related to ET-1 and NO in plasma and lung homogenates and CO in lung homogenate as well. The level of TNF-α increased after infusion of LPS into culture supernatant of Kupffer cells in vitro. However, TNF-α significantly decreased after pretreatment with glycine, PD98059 and SB212850. Glycine could antagonize the effect of LPS in vivo and in vitro.
CONCLUSION: Intestinal endotoxemia accompanying by cirrhosis may be an important mechanism in the development of hepatopulmonary syndrome in rats. Overproduction of TNF-α due to endotoxin stimulation of Kupffer cells via mitogen-activated protein kinase (MAPK) signal transduction pathway may be a major mechanism mediating the pathologic alterations of hepatopulmonary syndrome.
- Citation: Zhang HY, Han DW, Wang XG, Zhao YC, Zhou X, Zhao HZ. Experimental study on the role of endotoxin in the development of hepatopulmonary syndrome. World J Gastroenterol 2005; 11(4): 567-572
- URL: https://www.wjgnet.com/1007-9327/full/v11/i4/567.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i4.567
Hepatopulmonary syndrome (HPS) is characterized by liver disease, hypoxemia, increase of alveolar-arterial gradient, and intrapulmonary vascular dilatation[1-5]. HPS may complicate several forms of chronic liver disease. Cirrhosis is by far the commonest condition linked to HPS because of its high prevalence. To diagnose HPS, patients must demonstrate an elevated alveolar-arterial oxygen gradient (>20 mmHg) and evidence of intrapulmonary vascular dilatations by contrast-enhanced “bubble” echocardiography or radionuclide perfusion scanning. The physical signs most closely linked to HPS in liver patients include cyanosis, digital clubbing, and cutaneous spider nevi.
Hypoxemia is one of the three characteristics of HPS. Overall, hypoxemia is seen in about one-third of patients with cirrhosis[6,7]. The pathological basis of hypoxemia may ascribe to intrapulmonary vasodilatation. Its primary pathogenesis is as follows: (1) the alveolar ventilation - perfusion mismatching, (2) diffusion impairment or the alveolar-capillary oxygen disequilibrium and (3) the right-left shunting of blood vessels. A growing body of researchers has focused on NO in recent years. Exhaled NO level is elevated in patients and animals with HPS, indicating increased production of NO in lungs, which might be the cause of intrapulmonary vasodilatation and HPS[8,9], and increased NO may result from ET-1 overproduction[10]. An enhanced level of ET-1 has been observed in experimental and human cirrhosis. The elevated ET-1 levels in plasma are correlated with increased endothelial nitric oxide synthase (eNOS) levels in the pulmonary microcirculation and arterial gas exchange abnormalities[9]. Moreover, hemoglobin has a bioregulatory role for NO[11]. Early studies have proposed that hemoglobin acts solely as a sink limiting NO access to vascular smooth muscle and opposing its vasodilatation role and inactivating it[12]. An emerging hypothesis is that NO additionally functions as a hormone[12]. More recent work has shown that hemoglobin functions as both a reservoir and a vehicle for NO bioactivity[13-15]. It could regulate bioactivity of NO by O2 partial pressure-dependent allosteric transition of hemoglobin between its“tense-state and relaxed-state”. It is probable that capture of NO bioactivity occurs in highly oxygenated tissues and release, in relatively ischemic tissues.
Produced from hemeoxygenase-1 (HO-1, also known as hsp32), CO is comparable with NO, possessing an important bioactivity. The activity of HO-1 can be induced by various stimuli that have the capacity of provoking oxidative stresses, such as hyperthermia, hypoxia, endotoxin, ischemic/reperfusion injury, and radiation, and is considered to be one of the most sensitive indicators of cellular injury[16,17]. There is evidence that overexpression of HO-1 is associated with potent cytoprotective effects[16-18]. Recent studies have shown that up-regulation of HO-1 expression exerts important adaptive antioxidant and anti-inflammatory functions in cellular protection from pathophysiological conditions[16,17]. Investigations have shown that the balance between Ho-1/CO and NOS/NO systems plays important biological roles in maintaining homeostasis of living organisms. But the pathophysiological functions of CO and NO, and their mutual relations in development of HPS have not been elucidated as yet.
We successfully induced hepatic encephalopathy and hepatorenal syndrome in rats with cirrhosis by employing a small dosage of LPS, and demonstrated that their occurrence had a close correlation with intestinal endotoxemia(IETM)[19-21]. However, it is not clear whether endotoxin development influences of HPS. The present study was to explore the effect of IETM on the pathogenesis of HPS.
Male Wistar rats, weighing 230-280 g, obtained from the Animal Center of Shanxi Medical University, were employed in the present study. All animals received humane care during the study under a protocol that was in accordance with institutional guidelines for animal research and was approved by the Ethics and Research Committee of Shanxi Medical University. Lipopolysaccharide (LPS, from Escherichia coli sterotype 0111:B4) was purchased from Sigma Chemical Co. PD98059 (2’-amino-3’-methoxyflavone) and SB202190 (FHPI) were from A.G.Scientific, Inc. ET-1 and TNF-α radioimmunoassay kits were provided by Radioimmunological Institute of PLA General Hospital. NO detection kit was obtained from Nanjing Jiancheng Bioengineering Institute. Hou reagent, for determination of endotoxin in plasma, was provided by Shanghai Yi Hua Scientific, Inc. Other reagents used in the present study were all of analytical grade.
The preparation of rat model of cirrhosis was described in the literature[22]. Briefly, cirrhosis was induced in rats by subcutaneous administration of 400 g/L CCL4 oil solution, promoted by a diet containing high cholesterol and alcohol, low protein and choline. Cirrhosis developed in the sixth week. At the eighth week, cirrhosis developed into its advanced stage. The experimental rats were randomly divided into five groups: normal control, normal rats + a small dosage of LPS, cirrhosis, cirrhosis + a small dosage of LPS,and cirrhosis + glycine + a small dosage of LPS groups. At end of the 8th wk, rats were treated with 300 μg LPS/100 g.body weight and 1 g/rat of glycine about 4 h prior to LPS. Three hour after LPS treatment, blood and tissues were collected for various measurements.
All experimental rats were fasted overnight. Animals were fixed on the operation table in conscious state to record respiratory curve. Blood was collected for gas analysis under sterile condition.
Endotoxin, TNF-α, ET-1, NO and CO levels in plasma were measured according to manufacturer’s instructions of the kits. For determination of ET-1, NO and CO in pulmonary tissue, tissue samples were homogenized in ice-cold physiological saline, then centrifuged at 3000 r/min for 10 min at 4 °C. The resulting supernatant was stored at -70 °C until analysis.
Samples from the left lobe of lungs were collected into 100 g/L phosphate-buffered formaldehyde and fixed overnight. Serial 4 μm thick sections were prepared after the samples were dehydrated in graded ethanol solutions, cleared in chloroform, and embedded in paraplast. A staining was performed with hematoxylin and eosin (HE) and studied under a light microscope in a blind fashion.
The rats were anesthetized, and the abdominal skin was shaved and sterilized with an iodine solution. Mesenteric lymph nodes were dissected, crushed, plated onto chocolate agar plates containing blood, and incubated at 37 °C for 24 h. The number of colony forming units was counted and Gram staining in cirrhosis was compared with normal control.
Thirty minutes after the injection of 200 μ Ci of 99mTc-labeled albumin macroaggregates into the tail vein of experimental rats, the ratio of brain-over-lung radioactivity was determined.
Kupffer cells were isolated from male Wistar rats, using the standard techniques of collagenase perfusion, followed by differential centrifugation using Percoll[23]. Briefly, liver was perfused in situ via the portal vein with Hanks’ balanced salt solution, followed by Hanks, solution with 0.5 g/L collagenase. The liver was then excised and minced before incubation with Hanks/collagenase solution with continuous stirring at 37 °C for 60 min. The liver slurry was filtered through gauze mesh and washed with culture media and centrifuged at 50 r/min for 3 min twice. The resulting supernatant was collected and washed and centrifuged at 500 r/min for 10 min. Cells were resuspended and further purified using a discontinuous gradient of 250 mL/L and 600 mL/L Percoll. Purified non-parenchymal cells were washed and cultured in media containing Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 15 mmol/L HEPES, and 10-6 mol/L insulin at 37 °C for 2 h. Kupffer cells were enriched and cells (1×106/plate) were seeded on uncoated plastic tissue culture dishes and cultured in DMEM supplemented with 100 mL/L fetal calf serum (FCS), 2 mmol/L L-glutamine and standard antibiotics in a humidified atmosphere containing 950 mL/L air and 50 mL/L CO2 at 37 °C for 36-72 h for the experiments.
All dishes were divided into 5 groups: (1) normal control, without any treatment;(2) endotoxin group, infusion of 100 ng/mL LPS into dishes;(3) PD98059 group, addition of 10 μmol/L PD98059 at 30 min prior to LPS; (4) SB202850 group, addition of 2 μmol/L SB212850 at 30 min prior to LPS, and (5) glycine group, addition of 10 mmol/L glycine at 30 min prior to LPS.
Supernatant was harvested at 3 h after addition of LPS for measurement of TNF-α. Each group included 2 dishes and repeated 6 times.
Results were evaluated using analysis of variance and correlated by SPSS10.0 software. All values expressed as mean±SD. P values less than 0.05 were considered statistically significant.
In normal rats, the rhythmic breathing and regular curves were recorded. Respiratory frequency and range were appropriate. But, a slowed and deepened breath with occasional pause was observed in rats with cirrhosis. After administration of a given dosage of LPS in normal and cirrhotic rats, the frequency became slightly quicker, indicating an existence of hyperventilation. Glycine could antagonize the effect of LPS.
In cirrhotic rats, pH was in normal range. Partial pressures of O2 and CO2 in arterial blood (PaO2, PaCO2) and SB in arterial blood decreased. Arterial O2 and SB fell markedly. There was a close correlation between O2 and determined endotoxin. Metabolic acidosis accompanying respiratory alkalosis was primary type of acid-base imbalance. The alveolar arterial oxygen gradient was sharply widened. After administration of a given dose of LPS, respirations worsened. Pretreatment of glycine could alleviate various pathological alterations (Tables 1, 2 and 3).
| Group | n | pH | PaCO2 (mmHg) | PaO2 (mmHg) |
| Normal control | 8 | 7.38±0.03 | 33.94±4.21 | 94.71±13.72 |
| Normal+endotoxin | 7 | 7.31±0.02 | 28.13±2.56 | 117.51±22.30 |
| Cirrhosis | 7 | 7.34±0.14 | 28.76±5.57 | 88.87±28.46 |
| Cirrhosis+endotoxin | 6 | 7.32±0.04 | 24.98±8.69 | 112.55±24.02 |
| Cirrhosis+endotoxin+glycine | 6 | 7.31±0.10 | 33.25±5.10 | 110.40±12.71 |
| Group | n | O2cont (mL/dL) | SB (mmol/L) | AB (mmol/L) | AaDO2 |
| Normal control | 8 | 20.40±0.32 | 20.76±1.55 | 19.61±2.17 | 3.44±3.26 |
| Normal+endotoxin | 7 | 20.63±0.34 | 15.96±1.06 | 13.77±1.30c | |
| Cirrhosis | 7 | 19.80±1.1a | 18.00±4.93 | 15.89±5.05c | 9.9±2.08e |
| Cirrhosis+endotoxin | 6 | 20.58±0.27 | 15.10±2.33 | 12.38±3.68c | 18.48±7.86eg |
| Cirrhosis+endotoxin+glycine | 6 | 20.60±0.14 | 17.63±3.59 | 16.43±3.82 |
| Group | n | State of acid-base | Ratio of imbalance | ||
| Normal | MA | MA+RA | |||
| Normal control | 8 | 8 | 0/8 | ||
| Normal+endotoxin | 7 | 6 | 1 | 7/7 | |
| Cirrhosis | 7 | 1 | 6 | 7/7 | |
| Cirrhosis+endotoxin | 6 | 2 | 4 | 6/6 | |
| Cirrhosis+endotoxin+glycine | 6 | 2 | 2 | 2 | 4/6 |
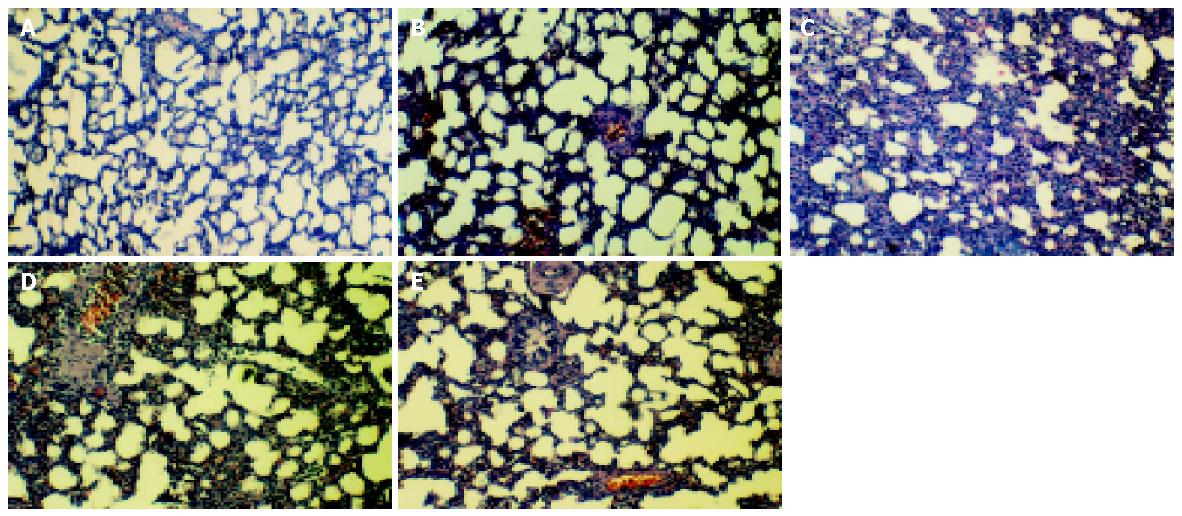
Histology with hematoxylin and eosin staining showed lymphoid aggregates and massive accumulation of giant macrophages in the alveolar spaces and its walls, and widened alveolar wall architectures were observed in cirrhotic rats. After administration of a given dosage of LPS, alveolar wall further widened with decreased density of cells and narrowed alveolar space, infiltration of polymorphs and fibrinous exudates, indicating interstitial pulmonary edema and inflammatory reaction. There was severe stasis of blood in alveolar walls and numerous red cells extravasated airspace indicating the widespread dilatation of alveolar capillaries and the increase of permeability of microvasculature. However, when normal rats were administered a given dosage of LPS, there was only a mild pathological change. Pretreatment of glycine could alleviate various pathological changes (Figure 1: A-E).
The goal of present study was to identify whether bacteria translocation could occur by determining colony numbers of bacteria in the mesenteric lymph nodes so as to further judge whether bacteremia and IETM occured. The results showed that the number of colonies of bacteria in rats with cirrhosis was markedly higher than that in normal control and Gram-negative bacteria (Table 4).
| Group | Minor colony | Major colony | Gram reaction: |
| Normal control | 5 | 0 | gram-negative |
| Cirrhotic rats | 2 | 3 | predominantly |
Technetium 99m-labeled macroaggregated albumin had a diameter about 20-50 μm and was trapped in normal pulmonary capillary bed with a diameter of 8-15 μm but could pass through dilated pulmonary capillaries. The results demonstrated that the ratio of Tc99m-MAA brain-over-lung radioactivity significantly raised in cirrhotic rats, indicating intrapulmonary vasodilatation. The manifestations were exacerbated after injection of LPS. The pulmonary microcirculation tended to increase after administration of LPS in normal rats (Table 5). The ratio of brain-over-lung radioactivity was correlated with the plasma endotoxin level.
The endotoxin and TNF-α change in plasma was similar. They rose obviously in rats with cirrhosis and further increased after addition of a given dosage of LPS. The pretreatment of glycine could alleviate various pathological alterations (Table 6). There was a close correlation between endotoxin and TNF-α levels in plas.
Changes of ET-1, NO and CO in plasma and lung homogenates are summarized in Tables 7, 8 and 9. Except CO content in plasma, the changing patterns were comparable in endotoxin and TNF-α. There was no obvious difference in plasma CO among various groups. Level of endotoxin and TNF-α in plasma was correlated with that of ET-1 and NO in plasma and lung homogenates and CO in lung homogenate.
| Group | Plasma (ng/L) | Lung homogenate (ng/kg protein) |
| Normal control | 92.0083±7.7498 | 163.3167±30.8496 |
| Normal+endotoxin | 391.3117±74.8649a | 290.4733±78.3474e |
| Cirrhosis | 224.0433±8.1965a | 303.3683±44.6253e |
| Cirrhosis+endotoxin | 377.2550±28.9485a | 385.5517±143.1575e |
| Cirrhosis+endotoxin+glycine | 271.2217±43.7350ac | 361.2283±71.4878eg |
TNF-α was an indicator of the secretory function of Kupffer cells in the present experiment. The findings showed that TNF-α significantly increased after LPS infusion into culture supernatant. In addition, TNF-α was significantly decreased after pretreatment with glycine, PD98059 and SB212850 (Table 10).
Normal acid-base balance in blood is dependent on efficient alveolar ventilation and perfusion, as well as successful gas exchange. This leads to the normal PaO2 and PaCO2, and normal blood pH. In our experiment, a deepened and slowed breath, decreased PaO2 and PaCO2, and O2 and increased alveolar-arterial oxygen gradient were seen in rats with cirrhosis at the end of the eighth week, indicating the presence of hypoxia and disturbance of acid-base as a result of the gas exchange abnormality in lung. The ratio of Tc99m-MAA brain-over-lung radioactivity was elevated significantly in cirrhotic rats, which showed that a portion of technetium-99 macroaggregated albumin particles traversed the pulmonary capillary bed and deposited in systemic microvascular beds, thereby suggesting the intrapulmonary vasodilatation in predominant areas of alveoli. These changes were in accordance with manifestations of the patients with HPS. Therefore, these results demonstrate the presence of classical HPS in cirrhotic rats.
The mononuclear-phagocyte system of the liver is important in clearing organisms from the portal circulation. During genesis of cirrhosis, the drained portal blood is obstructed, leading to swollen mucosa and weakened intestinal movements and decreased secretion of bile, which bring about the massive bacterial overgrowth in the lumen of the bowel, particularly a profusion of Gram-negative enteric organisms and the production of endotoxin. On the other hand, mucosal barrier function decreases, deterioration of hepatocytes and Kupffer cells occurrs, and portal-systemic shunts form, which cause the invasion of enteric organisms/endotoxin into blood and the formation of bacteremia and IETM. Endotoxin itself in turn impairs mitochondria and lysosome in enteric epithelial cells with cell autolysis. Ultimately, a vicious cycle is formed between IETM and the permeability of enteric mucosa. Our findings indicate that increased levels of endotoxin in plasma and a rising number of colonies of the organisms in mesenteric lymph nodes with Gram-negative dyeing, fully demonstrate the presence of bacteremia and IETM in genesis of HPS. The level of plasma endotoxin is significantly correlated with both a sharply decreased O2 and an evidently increased ratio of brain-over-lung radioactivity, suggesting that IETM is closely related to both hypoxia and intrapulmonary vasodilatation in cirrhosis.
In recent years, more attention has been paid to the role of ET-1 in HPS. A working model of the potential effects of ET-1 on the pulmonary microcirculation has been established. Under normal conditions, ET-1 is a paracrine vasoconstrictor that regulates vascular tone[24]. It is released from vascular endothelial cells, predominantly in an abluminal direction, where it targets the endothelin A receptor on vascular smooth muscle cells and initiates vasoconstriction[24]. To a lesser degree, ET-1 released into the lumen targets the endothelin B receptor on endothelial cells and triggers nitric oxide production, which counterbalances vasoconstrictive effects[24]. After hepatic injury, ET-1 from the liver reaches the pulmonary circulation and may preferentially interact with the endothelin B receptor on the luminal surface of the pulmonary vascular endothelium. In this setting, ET-1 might act as an endocrine vasodilator triggering enhanced endothelial nitric oxide production and intrapulmonary vasodilatation. In order to further explore the role of endotoxin in the pathogenesis of HPS, we designed a group by administering a small dosage of exogenous bacterial LPS so as to increase the levels of plasma endotoxin in cirrhotic rats, mimicking possibly aggravating endotoxemia clinically in the end-stage of chronic hepatic diseases due to precipitating factors. In addition, we designed two groups of normal rats injected with bacterial LPS and control rats injected with bacterial LPS antagonized with glycine. A massive accumulation of giant macrophages in the alveolar spaces and its walls, raised ET-1 and NO in pulmonary homogenates, and increased ET-1 and NO and TNF-α in plasma of the rats with cirrhosis were observed. Plasma level of endotoxin was found to be markedly correlated with these factors. TNF-α was also found to be related to ET-1 and NO. The changes of all indicators were further aggravated after the rats were injected with a given dosage of bacterial LPS demonstrating the action of IETM on it. The pretreatment of glycine could strikingly antagonize the effect of bacterial LPS, suggesting that ET-1 and NO play a role in the development of HPS. The changes of ET-1 and NO were found to be closely related to endotoxin demonstrating the important role of IETM in genesis of HPS. It was reported that bacterial LPS could stimulate the release of ET-1 in human umbilical vein endothelial cells that locate at the post-transcriptional level[25]. Hypoxia could also provoke the release of ET-1 in lung[26]. Together with our results that decreased O2 correlated with plasma ET-1, it is suggested that the lung might be a main resource of ET-1 besides the liver. Therefore, we could deduce that IETM might irritate Kupffer cell release of TNF-α directly and/or indirectly inducing the production of ET-1. The latter successively mediates the secretion of NO in its endocrine manner to contribute to HPS. It has been demonstrated that an inhibitor of NO synthesis (L-NAME) normalizes alveolar arterial oxygen gradient, brain radioactivity, pulmonary vascular resistance, reactivity to hypoxia and angiotensin II, exhaled NO, and NOS activities in rats with common bile duct ligation[27]. This is an important evidence about the role of NO in HPS. But, it is necessary to elucidate the significance of massive accumulation of macrophages in pulmonary architecture.
In rats with cirrhosis, we observed the increased level of CO in pulmonary homogenates, but no evident alterations of CO in plasma in our experiment. Still, no marked changes of plasma CO in other groups were seen. But, increased TNF-α in plasma was correlated with CO in lung homogenates, suggesting that the overproduction of TNF-α in injured liver may be an important cause for the production of CO in lung. But, we could not find the correlation between CO and NO. However, the functions of increased CO and decreased NO in pulmonary homogenates were noted by observing the effect of Da Huang Su in our experiment. In addition, NO is related to malondialdehyde(MDA), a product of lipid peroxidation of biomembrane. These results might suggest NO to be an injury factor and CO, a protective factor in the course of genesis of HPS. Therefore, it is of great importance to further study the relation between CO and NO and their significance in HPS.
Our previous investigations showed that glycine could down-regulate the receptor (CD14) of bacterial LPS on surface of Kupffer cells, and inhibit the activity of nuclear factor kappa B(NF-κB)[28-30]. Furthermore, we observed the effect of endotoxin on MAPK signal transduction system in cultured Kupffer cells in vitro. The experimental results indicate that exposure to bacterial LPS in cultured Kupffer cells in vitro could notably increase the secretion of TNF-α, and glycine could antagonize bacterial LPS to reduce the production of TNF-α. The efficacy of glycine mimics PD98059 (an inhibitor of ERK) and SB212850 (an inhibitor of p38MAPK). These findings further demonstrate that the overproduction of TNF-α by endotoxin-stimulated Kupffer cells via MAPK signal transduction pathway may be the molecular mechanism that leads to the development of HPS.
In conclusion, IETM accompanying cirrhosis may be an important mechanism in the development of HPS in rats. The overproduction of TNF-α due to endotoxin stimulated Kupffer cells via MAPK signal transduction pathway may be a major mechanism mediating the pathologic alterations of HPS.
Edited by Kumar M and Wang XL Proofread by Zhu LH
| 1. | Krowka MJ, Cortese DA. Hepatopulmonary syndrome. Current concepts in diagnostic and therapeutic considerations. Chest. 1994;105:1528-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 160] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Krowka MJ. Clinical management of hepatopulmonary syndrome. Semin Liver Dis. 1993;13:414-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Rodríguez-Roisin R, Agustí AG, Roca J. The hepatopulmonary syndrome: new name, old complexities. Thorax. 1992;47:897-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 163] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 4. | Castro M, Krowka MJ. Hepatopulmonary syndrome. A pulmonary vascular complication of liver disease. Clin Chest Med. 1996;17:35-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 123] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Stoller JK. As the liver goes, so goes the lung. Chest. 1990;97:1028-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Sherlock S. The liver-lung interface. Semin Respir Med. 1988;9:247-253. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Hourani JM, Bellamy PE, Tashkin DP, Batra P, Simmons MS. Pulmonary dysfunction in advanced liver disease: frequent occurrence of an abnormal diffusing capacity. Am J Med. 1991;90:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 66] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Cremona G, Higenbottam TW, Mayoral V, Alexander G, Demoncheaux E, Borland C, Roe P, Jones GJ. Elevated exhaled nitric oxide in patients with hepatopulmonary syndrome. Eur Respir J. 1995;8:1883-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 129] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Rolla G, Brussino L, Colagrande P, Dutto L, Polizzi S, Scappaticci E, Bergerone S, Morello M, Marzano A, Martinasso G. Exhaled nitric oxide and oxygenation abnormalities in hepatic cirrhosis. Hepatology. 1997;26:842-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 103] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Luo B, Abrams GA, Fallon MB. Endothelin-1 in the rat bile duct ligation model of hepatopulmonary syndrome: correlation with pulmonary dysfunction. J Hepatol. 1998;29:571-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | McMahon TJ, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, Gow AJ, Pawloski JR, Watke P, Singel DJ. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8:711-717. [PubMed] |
| 12. | Lane P, Gross S. Hemoglobin as a chariot for NO bioactivity. Nat Med. 2002;8:657-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1257] [Cited by in RCA: 1181] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 14. | Gow AJ, Luchsinger BP, Pawloski JR, Singel DJ, Stamler JS. The oxyhemoglobin reaction of nitric oxide. Proc Natl Acad Sci USA. 1999;96:9027-9032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 320] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 15. | Pawloski JR, Hess DT, Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature. 2001;409:622-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 443] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 16. | Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1029-L1037. [PubMed] |
| 17. | Agarwal A, Nick HS. Renal response to tissue injury: lessons from heme oxygenase-1 GeneAblation and expression. J Am Soc Nephrol. 2000;11:965-973. [PubMed] |
| 18. | Coito AJ, Buelow R, Shen XD, Amersi F, Moore C, Volk HD, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 gene transfer inhibits inducible nitric oxide synthase expression and protects genetically fat Zucker rat livers from ischemia-reperfusion injury. Transplantation. 2002;74:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Han DW. Intestinal endotoxemia as a pathogenetic mechanism in liver failure. World J Gastroenterol. 2002;8:961-965. [PubMed] |
| 20. | Li XQ, Han DW, Xu RL, Zhao YC, MA XH. Hepatic encephalopathy in cirrhoticrats induced by endotoxin injection. Zhongguo Binglishengli Zazhi. 1999;15:151-153. |
| 21. | Han DW. IETM and liver diseases. Beijing: China Science and Technology Press 2003; 879-886. |
| 22. | Han DW, Ma XH, Zhao YC. Study of a model about animals with cirrhosis. Shanxi Yiyao Zazhi. 1979;4:1-6. |
| 23. | Smedsrød B, Pertoft H. Preparation of pure hepatocytes and reticuloendothelial cells in high yield from a single rat liver by means of Percoll centrifugation and selective adherence. J Leukoc Biol. 1985;38:213-230. [PubMed] |
| 24. | Filep JG. Endothelin peptides: biological actions and pathophysiological significance in the lung. Life Sci. 1993;52:119-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Ros J, Leivas A, Jiménez W, Morales M, Bosch-Marcé M, Arroyo V, Rivera F, Rodés J. Effect of bacterial lipopolysaccharide on endothelin-1 production in human vascular endothelial cells. J Hepatol. 1997;26:81-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Shirakami G, Nakao K, Saito Y, Magaribuchi T, Jougasaki M, Mukoyama M, Arai H, Hosoda K, Suga S, Ogawa Y. Acute pulmonary alveolar hypoxia increases lung and plasma endothelin-1 levels in conscious rats. Life Sci. 1991;48:969-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 104] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Nunes H, Lebrec D, Mazmanian M, Capron F, Heller J, Tazi KA, Zerbib E, Dulmet E, Moreau R, Dinh-Xuan AT. Role of nitric oxide in hepatopulmonary syndrome in cirrhotic rats. Am J Respir Crit Care Med. 2001;164:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Liu J, Han D, Xu R, Zhao Y. Effect of glycine on the expression of CD(14) gene and protein of hepatic tissue in the course of developing cirrhosis of rats. Zhonghua GanZangBing ZaZhi. 2002;10:181-184. [PubMed] |
| 29. | Chen XH, Wang F, Han DW, Xu RL, Zhao YC. Mechanism and effectsofglycine on endotoxin shock and endotoxin-induced hepatic injury. Zhonghua Shiyanwaike Zazhi. 2003;20:415-417. |
| 30. | Yun KM, Han DW, Xu RL, Zhao YC. Effect of LPS on phagocytosis of rat Kupffer cells in vitro. Zhongguo Binglishengli Zazhi. 2003;19:795-798. |