Published online Oct 21, 2005. doi: 10.3748/wjg.v11.i39.6085
Revised: April 8, 2005
Accepted: April 11, 2005
Published online: October 21, 2005
Patients with chronic hepatitis C virus (HCV) infection have a significantly increased prevalence of type 2 diabetes mellitus compared to controls or HBV-infected patients. Moreover, the incidence rate of post-liver transplantation diabetes mellitus (PTDM) also appears to be higher among patients with HCV infection. PTDM is often associated with direct viral infection, autoimmune disorders, and immunosuppressive regimen. Activation of tumor necrosis factor-α may be the link between HCV infection and diabetes. In this article, we reviewed the epidemiologic association between HCV infection and PTDM, highlighting the most recent pathophysiologic insights into the mechanisms underlying this association.
- Citation: Ma Y, Yan WW. Chronic hepatitis C virus infection and post-liver transplantation diabetes mellitus. World J Gastroenterol 2005; 11(39): 6085-6089
- URL: https://www.wjgnet.com/1007-9327/full/v11/i39/6085.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i39.6085
The last two decades have seen an increase in various types of organ transplantation for the treatment of end stage organ diseases. Prevention of organ rejection requires long-term immunosuppressive therapy, which places recipients at the increasing risk of infection and metabolic disorders, such as recurrent hepatitis C virus (HCV) infection and post-liver transplantation diabetes mellitus (PTDM). Recipients with HCV infection have a higher incidence of PTDM, which depends on the degree and duration of immunosuppressant[1,2]. On the other hand, the occurrence of PTDM is associated with direct viral infection, autoimmune disorders[3], and imm-unosuppressive regimen, notably tacrolimus[4]. PTDM characteristically shows insidious onset and aggressive behavior thereafter. PTDM in HCV-infected patients might be partially or completely recovered after reduction or switching of immunosuppressive therapy[5].
The high prevalence of diabetes mellitus, especially type 2 diabetes mellitus (type 2 DM) in HCV-infected patients comes from epidemiologic studies (Table 1), most of which are case control studies[6,7,9,10-11,13] in addition to two multicenters and cohort[8,12]. Risk factors resulting in the increasing prevalence of type 2 DM in HCV-infected patients include positive family history of diabetes and black ethnicity[14]. Thus, it is conceivable that HCV leads to type 2 DM in susceptible hosts. Immunogenetic studies suggest that an infectious process coexists in patients with type 2 DM and chronic liver disease and HLA-DR2, -DR51, -DQB6 haplotypes provide a two- and three-fold relative risk for the development of diabetes[15].
| PTDM (%) | RR (95%CI) | Study description | Reference | |
| HCV+ | HCV- | |||
| 32 | 12 | 3 (0.98-9.6)b | Case control | Zein et al 2000 |
| 34 | 9.8 | 0.95 (0.2-3.9)a | Case control | Bigam et al 2000 |
| 20.9 | - | 1.9 (0.4-14.2) | Multicenter | Khalili et al 2004 |
| 43 | 13 | 1.9 (1-2.6)b | Case control | AlDosary et al 2002 |
| 64 | 28 | 2 (0.9-4.6)b | Case control | Baid et al 2002 |
| 29 | 10 | 2 (0.96-4.3)b | Case control | Bigam et al 2000 |
| 72 | 37 | 4.8 (0.8-30)d | Case control | Yildiz et al 2002 |
| 62 | 8 | 11.6 (1.7-79)b | Cohort | Delgado-Borrego et al 2004 |
| 18.29 | - | - | Case control | Parolin et al 2004 |
It is reported that liver transplantation recipients with HCV infection have a four- to eight-fold prevalence of diabetes compared with recipients with other viral or cholestatic liver diseases 1 year after liver transplantation. A recent study of 260 HCV-infected patients has confirmed that insulin resistance is an independent predictor of the degree of fibrosis, and that insulin sensitivity has a significant correlation with serum aspartate aminotransferase, histological activity index and degree of fibrosis[17] in non-diabetic HCV-infected patients. Gray et al[16] observed that abnormal liver function tests are found in 72.3% of HCV-positive diabetic patients and in only 24.7% of HCV-negative diabetic patients (P<0.001). In summary, the occurrence of PTDM seems to be associated with HCV infection.
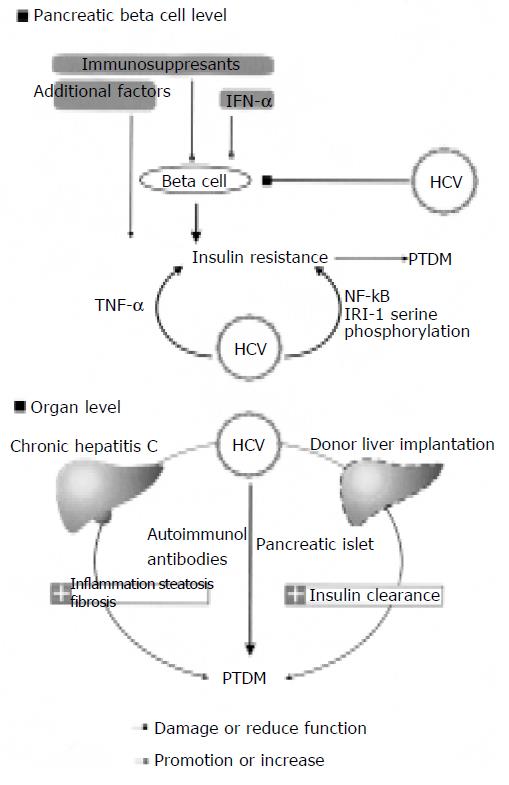
The pathophysiologic mechanism underlying the development of PTDM in patients with HCV infection has not been clearly illustrated. Possible mechanisms are shown in Figure 1.
A study of liver biopsy specimens from non-diabetic HCV-infected patients has revealed significant impairments in the insulin-signaling pathway[18] which is strikingly similar to the known effect of tumor necrosis factor-α (TNF-α) on insulin resistance[19]. The role of TNF-α in the pathogenesis of this HCV-associated insulin resistance state is strongly supported by findings of elevated intra-hepatic TNF-α and amelioration of the metabolic abnormalities after TNF-α antibodies are administered[2]. The interrelationship between HCV and other predisposing conditions of PTDM could partly be mediated by TNF-α and most of the established risk factors for PTDM, such as obesity, aging[20] black ethnicity[21] and a family history of type 2 DM[22].
HCV replicates in hepatocytes, but its genome has been identified in a number of other tissues, including pancreatic acinar cells and epithelial cells of the pancreatic duct[23]. The destruction of pancreatic b cells could be mediated either directly by HCV or by HCV-induced immune responses, but evidence is scanty[3]. More recently, a transgenic mouse model that specifically expresses the HCV core protein in hepatocytes has been studied[2]. These animals had insulin resistance at an early age, and when challenged by a high fat diet, they developed glucose intolerance. Further characterization of the metabolic defects in these animals revealed that insulin resistance was caused mainly by failure of insulin to suppress hepatic glucose production. Piquer et al[24] assessed the prevalence of islet cell autoantibodies in 303 non-selected HCV-infected patients (277 non-diabetic and 26 type 2 DM patients) and 273 sex- and age-matched control subjects, and found that patients with HCV have no significantly higher pancreatic autoantibodies compared with controls. However, it was reported that type 1 DM occurs more frequently in HCV patients treated with interferon (IFN) as a result of amplification of previously existing autoimmunity against pancreatic b cells, and that treatment of healthy volunteers with IFN can stimulate counter-regulatory hormone secretion, impair glucose tolerance and insulin sensitivity and induce insulin clearance, suggesting that HCV-induced immune response might be involved in the development of insulin resistance[25].
The overall effects of immunosuppression may potentiate the diabetogenic effects of HCV infection by enhancing the level of viral replication. A study on the effects of corticosteroids on post-transplant levels of viremia and PTDM showed that pulsed intravenous methylprednisolone therapy is associated with transient 4- to 100-fold rise in HCV RNA levels and the reduced translocation of glucose transporter 4 from cytosol into membrane, and that higher HCV RNA level is correlated with increased severity of graft and islet injury[28,43,44]. Immun-osuppressive agents, especially steroids and calcineurin inhibitors, are diabetogenic. The direct and indirect effects of corticosteroids, CyA on pancreatic b cells are well documented. With regard to FK506, the available data are not as extensive as for CyA, and its role in low blood insulin levels vs insulin resistance has not been fully evaluated. Human pancreatic allograft biopsies demonstrating both FK506 and CyA can induce structural damage of the graft islets, for example, cytoplasmic swelling, vacuolization, apoptosis, and abnormal immunostaining for insulin and the absent or reduced dense core secretory granules in the b cells. These changes are related to the doses of FK506 and/or CyA used. Although CyA and FK506 bind to different target molecules, both drugs inhibit or perturb the intermediate molecular events of insulin signal transduction (serine phosphorylations/dephosphorylations) in the same fashion. As a result, insulin signal fails to dephosphorylate the cytoplasmic component of the nuclear factor of activated signal transduction and transcription of insulin-sensitive gene, thus incorporating insulin resistance[26,19].
Nonalcoholic fatty liver disease is associated with type 2 DM and the metabolic syndrome in patients with HCV[27]. However, the role of HCV as a risk factor for fatty liver has been questioned. It was reported that obesity and hyperlipidemia have a confounding effect on the association between HCV and steatosis[28]. In one study, serum ferritin levels were determined in 123 HCV-infected patients (55 diabetic and 68 non-diabetic patients)[30]. Increased iron store, which occurs in up to 40% of patients with chronic HCV infection[29] has been linked to the pathogenesis of diabetes development in those patients. The hepatic parasympathetic nerves are mediated by the activation of the Ach/NO/cGMP pathway involved in the secretion of a hepatic insulin sensitizing substance to mediate peripheral insulin sensitivity[39,40]. Schneiter et al[31] found that liver transplantation recipients have severe insulinopenia as a result of the increased insulin clearance[31].
PTDM shares many similarities with type 2 DM in that the onset can be insidious, and individuals may experience glucose intolerance and may be asymptomatic for years[5]. The development of PTDM involves two distinct phases: (a) patients are initially at the greatest risk during the first 6 mo post-transplantation, and (b) the number of patients developing diabetes increases progressively thereafter. This has been illustrated by a study of 618 liver allograft recipients in which 7.2% of patients developed diabetes in the first 6 mo after transplantation, but then the percentage of cases increased linearly, leading to cumulative percentages of 7.1%, 10.4%, 13.2%, 20.5%, and 29.8%, respectively after 1, 3, 5, 10, and 15 years[32]. In a recent study, unadjusted cumulative incidences of diabetes 3, 12, and 36 mo after kidney transplantation were 15.6%, 25.6%, and 35.4% compared to 8.8%, 15.4%, and 23.4% in patients who were HCV negative at transplantation (P<0.0001)[33]. The potentially asymptomatic and/or transient nature of diabetes after transplantation makes the condition difficult to diagnose, thus underlining the importance of establishing a precise definition. It is recommended that the definition and diagnosis of PTDM should be based on the currently accepted definition of diabetes mellitus by the American Diabetes Association[5]. Diabetes mellitus is diagnosed by three fasting glucose measurements in the plasma above 7.0 mmol/L and/or antidiabetic medication (oral antidiabetic drugs or insulin), excluding periods requiring total parenteral nutrition or high steroid dosages for treatment of acute rejection, which may transiently impair glucose tolerance. After liver transplantation, patients with diabetes mellitus are classified either as non-insulin-requiring PTDM when taking only oral antidiabetic drugs or diet or as insulin-requiring PTDM when they require insulin injections. This classification of the patients should be done more than 1 year after liver transplantation[34]. Some preliminary reports suggest that pre-emptive therapy with IFN or a combination of IFN plus ribavirin may lead to less severe HCV infection recurrence after liver transplantation[41,42]. However, there are no controlled studies evaluating the prophylaxis of IFN in PTDM with HCV infection.
The HCV-diabetes association represents a major public health problem. Hundreds and thousands of patients in the USA alone are probably affected, and many more may have impaired glucose tolerance. Diabetes-related micro- and macrovascular complications are likely to occur, and the ongoing hepatic inflammatory response may contribute to atherogenesis. Furthermore, a putative bi-directional relationship between HCV-induced liver disease and diabetes may occur. Both insulin resistance and diabetes can adversely affect the course of chronic hepatitis C, and lead to enhanced steatosis, steatohepatitis, and liver fibrosis[35,36]. Moreover, recent evidence strongly suggests that steatosis and diabetes may also significantly enhance the risk of hepatocellular cancer[37,38].
There is evidence that HCV infection is related with the presence of PTDM. However, several questions remain to be answered. Firstly, randomized trials aimed to evaluate interactions of immunosuppressive regimens for the prophylaxis and treatment of PTDM are scarce. Secondly, the antiviral therapy for intervention remains to be determined. Finally, information is needed to clarify and diminish the effect of PTDM on quality of life and long-term outcomes. Further studies are necessary to evaluate the prognostic meaning of PTDM and the predictive factors for PTDM and to elucidate the pathophysiologic mechanisms.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Mathew JT, Rao M, Job V, Ratnaswamy S, Jacob CK. Post-transplant hyperglycaemia: a study of risk factors. Nephrol Dial Transplant. 2003;18:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 543] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 3. | Hadziyannis SJ. The spectrum of extrahepatic manifestations in hepatitis C virus infection. J Viral Hepat. 1997;4:9-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Kreis H, Oberbauer R, Campistol JM, Mathew T, Daloze P, Schena FP, Burke JT, Brault Y, Gioud-Paquet M, Scarola JA. Rapamune Maintenance Regimen trial. Long-term benefits with sirolimus-based therapy after early cyclosporine withdrawal. J Am Soc Nephrol. 2004;15:809-817. [RCA] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 189] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Davidson J, Wilkinson A, Dantal J, Dotta F, Haller H, Hernández D, Kasiske BL, Kiberd B, Krentz A, Legendre C. New-onset diabetes after transplantation: 2003 International consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation. 2003;75:SS3-S24. [PubMed] |
| 6. | Zein NN, Abdulkarim AS, Wiesner RH, Egan KS, Persing DH. Prevalence of diabetes mellitus in patients with end-stage liver cirrhosis due to hepatitis C, alcohol, or cholestatic disease. J Hepatol. 2000;32:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 172] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Bigam DL, Pennington JJ, Carpentier A, Wanless IR, Hemming AW, Croxford R, Greig PD, Lilly LB, Heathcote JE, Levy GA. Hepatitis C-related cirrhosis: a predictor of diabetes after liver transplantation. Hepatology. 2000;32:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Khalili M, Lim JW, Bass N, Ascher NL, Roberts JP, Terrault NA. New onset diabetes mellitus after liver transplantation: the critical role of hepatitis C infection. Liver Transpl. 2004;10:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | AlDosary AA, Ramji AS, Elliott TG, Sirrs SM, Thompson DM, Erb SR, Steinbrecher UP, Yoshida EM. Post-liver transplantation diabetes mellitus: an association with hepatitis C. Liver Transpl. 2002;8:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Baid S, Tolkoff-Rubin N, Farrell ML, Delmonico F, Williams WW, Hayden D, Ko D, Cosimi AB, Pascual M. Tacrolimus-associated posttransplant diabetes mellitus in renal transplant recipients: role of hepatitis C infection. Transplant Proc. 2002;34:1771-1773. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Yildiz A, Tutuncu Y, Yazici H, Akkaya V, Kayacan SM, Sever MS, Carin M, Karsidag K. Association between hepatitis C virus infection and development of post-transplantation diabetes mellitus in renal transplant recipients. Transplantation. 2002;74:1109-1113. [RCA] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Delgado-Borrego A, Casson D, Schoenfeld D, Somsouk M, Terella A, Jordan SH, Bhan A, Baid S, Cosimi AB, Pascual M. Hepatitis C virus is independently associated with increased insulin resistance after liver transplantation. Transplantation. 2004;77:703-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Parolin MB, Zaina FE, Araújo MV, Kupka E, Coelho JC. Prevalence of new-onset diabetes mellitus in Brazilian liver transplant recipients: association with HCV infection. Transplant Proc. 2004;36:2776-2777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Mehta SH, Brancati FL, Strathdee SA, Pankow JS, Netski D, Coresh J, Szklo M, Thomas DL. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003;38:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 287] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 15. | Bosi E, Minelli R, Bazzigaluppi E, Salvi M. Fulminant autoimmune Type 1 diabetes during interferon-alpha therapy: a case of Th1-mediated disease? Diabet Med. 2001;18:329-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Gray H, Wreghitt T, Stratton IM, Alexander GJ, Turner RC, O'Rahilly S. High prevalence of hepatitis C infection in Afro-Caribbean patients with type 2 diabetes and abnormal liver function tests. Diabet Med. 1995;12:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Hui JM, Kench JG, Chitturi S, Sud A, Farrell GC, Byth K, Hall P, Khan M, George J. Long-term outcomes of cirrhosis in nonalcoholic steatohepatitis compared with hepatitis C. Hepatology. 2003;38:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 294] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 18. | Aytug S, Reich D, Sapiro LE, Bernstein D, Begum N. Impaired IRS-1/PI3-kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology. 2003;38:1384-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 187] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Ruan H, Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev. 2003;14:447-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 350] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 20. | Ersoy C, Imamoğlu S, Budak F, Tuncel E, Ertürk E, Oral B. Effect of amlodipine on insulin resistance & amp; tumor necrosis factor-alpha levels in hypertensive obese type 2 diabetic patients. Indian J Med Res. 2004;120:481-488. [PubMed] |
| 21. | Kimball P, Elswick RK, Shiffman M. Ethnicity and cytokine production gauge response of patients with hepatitis C to interferon-alpha therapy. J Med Virol. 2001;65:510-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Kobayashi S, Suzuki M, Tsuneki H, Nagai R, Horiuchi S, Hagino N. Overproduction of N(epsilon)-(carboxymethyl)lysine-induced neovascularization in cultured choroidal explant of streptozotocin diabetic rat. Biol Pharm Bull. 2004;27:1565-1571. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Gowans EJ, Jones KL, Bharadwaj M, Jackson DC. Prospects for dendritic cell vaccination in persistent infection with hepatitis C virus. J Clin Virol. 2004;30:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Piquer S, Hernández C, Enriquez J, Ross A, Esteban JI, Genescà J, Bonifacio E, Puig-Domingo M, Simó R. Islet cell and thyroid antibody prevalence in patients with hepatitis C virus infection: effect of treatment with interferon. J Lab Clin Med. 2001;137:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Weir MR, Fink JC. Risk for posttransplant Diabetes mellitus with current immunosuppressive medications. Am J Kidney Dis. 1999;34:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 202] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Drachenberg CB, Klassen DK, Weir MR, Wiland A, Fink JC, Bartlett ST, Cangro CB, Blahut S, Papadimitriou JC. Islet cell damage associated with tacrolimus and cyclosporine: morphological features in pancreas allograft biopsies and clinical correlation. Transplantation. 1999;68:396-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 227] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 27. | Sanyal AJ, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Stravitz RT, Mills AS. Nonalcoholic fatty liver disease in patients with hepatitis C is associated with features of the metabolic syndrome. Am J Gastroenterol. 2003;98:2064-2071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Monto A, Alonzo J, Watson JJ, Grunfeld C, Wright TL. Steatosis in chronic hepatitis C: relative contributions of obesity, diabetes mellitus, and alcohol. Hepatology. 2002;36:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 236] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 29. | Riggio O, Montagnese F, Fiore P, Folino S, Giambartolomei S, Gandin C, Merli M, Quinti I, Violante N, Caroli S. Iron overload in patients with chronic viral hepatitis: how common is it? Am J Gastroenterol. 1997;92:1298-1301. [PubMed] |
| 30. | Furutani M, Nakashima T, Sumida Y, Hirohama A, Yoh T, Kakisaka Y, Mitsuyoshi H, Senmaru H, Okanoue T. Insulin resistance/beta-cell function and serum ferritin level in non-diabetic patients with hepatitis C virus infection. Liver Int. 2003;23:294-299. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Schneiter P, Gillet M, Chioléro R, Jéquier E, Mosimann F, Temler E, Téta D, Matter M, Wauters JP, Tappy L. Mechanisms of postprandial hyperglycemia in liver transplant recipients: comparison of liver transplant patients with kidney transplant patients and healthy controls. Diabetes Metab. 2000;26:51-56. [PubMed] |
| 32. | Huo TI, Yang WC, Wu JC, King KL, Lin CY, Loong CC, Lui WY, Chang FY, Lee SD. Long-term outcome of kidney transplantation in patients with hepatitis C virus infection. Hepatogastroenterology. 2001;48:169-173. [PubMed] |
| 33. | Duijnhoven EM, Boots JM, Christiaans MH, Wolffenbuttel BH, Van Hooff JP. Influence of tacrolimus on glucose metabolism before and after renal transplantation: a prospective study. J Am Soc Nephrol. 2001;12:583-588. [PubMed] |
| 34. | Stockmann M, Steinmüller T, Nolting S, Neuhaus P. Posttransplant diabetes mellitus after orthotopic liver transplantation. Transplant Proc. 2002;34:1571-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 773] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 36. | Castéra L, Hézode C, Roudot-Thoraval F, Bastie A, Zafrani ES, Pawlotsky JM, Dhumeaux D. Worsening of steatosis is an independent factor of fibrosis progression in untreated patients with chronic hepatitis C and paired liver biopsies. Gut. 2003;52:288-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 214] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 37. | Tazawa J, Maeda M, Nakagawa M, Ohbayashi H, Kusano F, Yamane M, Sakai Y, Suzuki K. Diabetes mellitus may be associated with hepatocarcinogenesis in patients with chronic hepatitis C. Dig Dis Sci. 2002;47:710-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Ohata K, Hamasaki K, Toriyama K, Matsumoto K, Saeki A, Yanagi K, Abiru S, Nakagawa Y, Shigeno M, Miyazoe S. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer. 2003;97:3036-3043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 245] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 39. | Lautt WW, Macedo MP, Sadri P, Takayama S, Duarte Ramos F, Legare DJ. Hepatic parasympathetic (HISS) control of insulin sensitivity determined by feeding and fasting. Am J Physiol Gastrointest Liver Physiol. 2001;281:29-36. |
| 40. | Guarino MP, Afonso RA, Raimundo N, Raposo JF, Macedo MP. Hepatic glutathione and nitric oxide are critical for hepatic insulin-sensitizing substance action. Am J Physiol Gastrointest Liver Physiol. 2003;284:G588-G594 45 Charlton M. Liver biopsy, viral kinetics, and the impact of viremia on severity of hepatitis C virus recurrence. Liver Transpl 2003; 9: S58-S62. |
| 41. | Sheiner PA, Boros P, Klion FM, Thung SN, Schluger LK, Lau JY, Mor E, Bodian C, Guy SR, Schwartz ME. The efficacy of prophylactic interferon alfa-2b in preventing recurrent hepatitis C after liver transplantation. Hepatology. 1998;28:831-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 153] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 42. | Singh N, Gayowski T, Wannstedt CF, Shakil AO, Wagener MM, Fung JJ, Marino IR. Interferon-alpha for prophylaxis of recurrent viral hepatitis C in liver transplant recipients: a prospective, randomized, controlled trial. Transplantation. 1998;65:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 116] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Charlton M. Liver biopsy, viral kinetics, and the impact of viremia on severity of hepatitis C virus recurrence. Liver Transpl. 2003;9:S58-S62. [PubMed] |
| 44. | Konrad T, Zeuzem S, Toffolo G, Vicini P, Teuber G, Briem D, Lormann J, Lenz T, Herrmann G, Berger A. Severity of HCV-induced liver damage alters glucose homeostasis in noncirrhotic patients with chronic HCV infection. Digestion. 2000;62:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.6] [Reference Citation Analysis (0)] |