Published online Sep 21, 2005. doi: 10.3748/wjg.v11.i35.5455
Revised: December 16, 2004
Accepted: December 20, 2004
Published online: September 21, 2005
AIM: Vascular endothelial growth factor (VEGF) is a potent mediator of peritoneal fluid accumulation following tumor progression. This study investigated the role of VEGF secreted by cancerous cells in the formation of malignant ascites.
METHODS: VEGF expression was eliminated by knockdown in the pancreas cancer cell-line PancO2 using vector-based short-hairpin type RNA interference (RNAi). Malignant ascites formation in the mouse was analyzed by intraperitoneal injection of PancO2 cells expressing VEGF or with expression knockdown.
RESULTS: The VEGF knockdown PancO2 cell was successfully established. Knockdown of VEGF did not affect cancer cell proliferation in vitro or in vivo. The volume of ascites following peritoneal expansion of the tumor in VEGF knockdown cells and control cells did not differ statistically in this in vivo study. Moreover, the VEGF concentration in the ascites did not differ statistically.
CONCLUSION: Malignant ascites formation might be mediated by VEGF production in noncancerous tissues, such as stromal compartments. An anti-VEGF strategy against malignant ascites could be applied to various tumors regardless of whether they secrete VEGF.
- Citation: Guleng B, Tateishi K, Kanai F, Jazag A, Ohta M, Asaoka Y, Ijichi H, Tanaka Y, Imamura J, Ikenoue T, Fukushima Y, Morikane K, Miyagishi M, Taira K, Kawabe T, Omata M. Cancer-derived VEGF plays no role in malignant ascites formation in the mouse. World J Gastroenterol 2005; 11(35): 5455-5459
- URL: https://www.wjgnet.com/1007-9327/full/v11/i35/5455.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i35.5455
Ascites formation is a critical problem in patients with advanced pancreatic cancer. Vascular endothelial growth factor (VEGF), known as an angiogenic growth factor, is a potent mediator of peritoneal fluid accumulation in tumors. The accumulation of tumor ascites fluid may result from increased permeability of the blood vessels lining the peritoneal cavity. The hyperpermeability of vessels might be mediated by VEGF secreted from tumor cells[1,2]. VEGF antisense plasmid suppresses ascites formation in ovarian carcinoma[3]. VEGF is widely expressed in various normal tissues and many tumors[4,5]. VEGF expression has been detected in both cancer cells themselves and stromal compartments[6-8], raising the question of the relative role of each compartment in VEGF-mediated ascites formation. Recent studies using RAS-transformed VEGF-deficient adult dermal fibroblasts or fibrosarcomas generated from VEGF-null RAS-transformed mouse embryonic fibroblasts concluded that VEGF production in stromal compartments plays a critical role in tumor angiogenesis[9,10]. However, the role of VEGF in ascites formation in the stroma is poorly understood.
RNA interference (RNAi) is an evolutionarily conserved mechanism of gene silencing. Recently, 21- to 23-nucleotide double-stranded RNA (short interfering RNA, siRNA) molecules have been shown to exhibit specific RNAi characteristics. A plasmid vector expressing siRNA was reported to enable long-term persistence of the silencing effect; a phenomenon termed as ‘stable RNAi’[11-13]. This system is a powerful tool for analyzing endogenous gene silencing.
To confirm whether VEGF secreted from cancer cells has a critical role in malignant ascites formation, we used the stable RNAi system to knockdown the VEGF gene in a mouse pancreatic cancer cell line.
pcDNA-Flag-VEGF expressing vector was constructed as follows: The total RNA was extracted from mouse Colon26 cell using ISOGEN (Nippon Gene, Tokyo, Japan), then the cDNA templates were synthesized from 1 μg of total RNA using the ImProm-IITM Reverse Transcription System (Promega). PCR amplification of mouse VEGFA (191 amino acids) containing Flag sequence was performed using the following two primers: 5’-ATG GAC TAC AAG GAC GAC GAT GAC AAA GAT AAC TTT CTG CTC TCT TGG GTG-3’ and 5’-TCA CCG CCT TGG CTT GTC ACA-3’. The PCR product was subcloned into the pCR4Blunt-TOPO vector (Invitrogen, USA), and then EcoRI-Flag-VEGF-EcoRI fragment was inserted into the EcoRI site of the pcDNA vector (Invitrogen, USA).
Plasmid carrying RNAi targeted to VEGF was constructed as previously described[12]. Five 21-nucleotide sequences of the mouse VEGF gene, which had no homology to genomic sequences in a BLAST search, were originally selected using the original algorithm[12]. We designed forward and reverse short-hairpin RNA (Table 1), and both oligonucleotides were annealed to each other (99 °C for 2 min, 72 to 4 °C for 2 h) and inserted into the BspMI site of the pcPUR+ U6icassette vector, thereby generating pcPUR+U6-VEGFi.
| Site 1 | Sense 5’-caccgagatagagtatatctttaaggtgtgctgtcccttgaagatgtactctatctcttttt-3’ |
| Antisense 5’-gcataaaaagagatagagtacatcttcaagggacagcacaccttaaagatatactctatctc-3’ | |
| Site 2 | Sense 5’-caccgagtatatcttcaagttgttcgtgtgctgtccggacggcttgaagatgtactcttttt-3’ |
| Antisense 5’-gcataaaaagagtacatcttcaagccgtccggacagcacacgaacaacttgaagatatactc-3’ | |
| Site 3 | Sense 5’-caccattatgaactttctgttctctgtgtgctgtccagagagcagaaagttcatggtttttt-3’ |
| Antisense 5’-gcataaaaaaccatgaactttctgctctctggacagcacacagagaacagaaagttcataat-3’ | |
| Site 4 | Sense 5’-caccatggatgtctattagtgaagcgtgtgctgtccgcttcgctggtagacgtccatttttt-3’ |
| Antisense 5’-gcataaaaaatggacgtctaccagcgaagcggacagcacacgcttcactaatagacatccat-3’ | |
| Site 5 | Sense 5’-caccaagttactgttgtctaattgagtgtgctgtcctcaattggacggcagtagcttttttt-3’ |
| Antisense 5’-gcataaaaaaagctactgccgtccaattgaggacagcacactcaattagacaacagtaactt-3’ |
PancO2 cell line[14] was generated from pancreatic cancer cells derived from C57BL/6 mice, obtained from Dr. Michael A. Hollingsworth, Eppley Institute, University of Nebraska, Omaha, NE, USA. The cells were maintained in Dulbecco’s modified Eagle’s medium (Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (Sigma) at 37 °C in a 50 mL/L CO2 atmosphere.
Stable knockdown cells were established as described[13]. Briefly, the targeting or control vectors were transfected into PancO2 cells using FuGene6 (Roche), and the puromycin-resistant clones or cell pools were selected as stable transfectants. We called stable clones transfected with pcPUR+U6-VEGFi or pcPUR+U6-iRenilla (control vector) as “VEGF-KD” or “VEGF-WT”, respectively.
Western blot analysis was performed as described[15]. Briefly, cells were lysed in RIPA buffer (50 mmol/L Tris pH 8, 0.1% SDS, 0.5% deoxycholate, 1% NP-40, 150 mmol/L NaCl, 1 tablet complete mini protease inhibitor/10 mL, Roche Diagnostics GmbH, Germany). Next, equal amounts of total cell lysates were electrophoresed and transferred to Pall Fluoro Trans W membrane (Wako, Osaka, Japan). The membranes were incubated with anti-Flag (M2) antibody or anti-β-actin antibody. After washing, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody and visualized using an enhanced chemiluminescence detection system (ECL Advance, Amersham Bioscience, UK).
To analyze the VEGF production of VEGF-WT and VEGF-KD clones, they were grown in six-well plates with standard medium for 24 h. The medium was replaced with 1 mL of fetal bovine serum-free medium and cells were grown for another 24 h. Then, the VEGF content of the culture supernatants was measured using ELISA (SRL, Japan).
3×104 VEGF-KD7 and VEGF-WT cells were seeded in 24-well plates, and the numbers of viable cells were determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma, St. Louis, MO, USA) at 24 and 48 h[16]. The absorbance at 570 nm was measured using a microplate reader.
C57BL/6 mice were purchased from CLEA (Japan). All procedures involving experimental animals were performed in accordance with protocols approved by the Committee for Animal Research of the University of Tokyo and complied with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86-23, revised 1985). 1×106 VEGF-KD7 or VEGF-WT cells were injected intraperitoneally into C57BL/6 mice. Ascites accumulation and peritoneal dissemination occurred approximately 2 wk following inoculation of the cancer cells. In this model, the tumor-bearing mice survived for 30-40 d. We weighed the mice every 2-3 d. Twenty-four days after inoculation, some of the mice were anesthetized with pentobarbital sodium (120 mg/kg) and the ascites collected. After centrifuging to remove cancer cells, the volume of ascites was measured. The VEGF concentration was measured using ELISA (SRL, Japan).
The data are expressed as mean±SD. Groups were compared using Student’s t-test. P<0.05 was considered statistically significant.
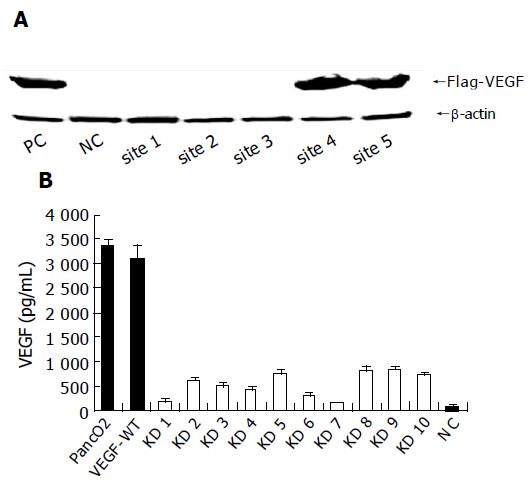
To study whether VEGF secreted from cancer cells has a critical role in the formation of malignant ascites in pancreatic cancer, we attempted to knockdown the VEGF gene in the mouse pancreatic cancer cell line PancO2. We constructed five plasmids that expressed short hairpin RNAs (as shown in Table 1) targeting the non-isoform-specific common area of VEGF (GenBank locus: NM 009505) under the control of the U6 promoter (pcPUR+U6-VEGFi1-5)[12]. Next, we transiently transfected PancO2 cells with pcPUR+U6-VEGFi1-5 and the Flag-VEGF expression vector, and examined VEGF expression using Western blotting. As shown in Figure 1A, of the five different sites tested, RNAi1-3 proved to be effective for blocking VEGF protein expression, and site 1 was used in subsequent experiments.
To establish stable VEGF knockdown cells, PancO2 cells were transfected with pcPUR+U6-VEGFi1 or pcPUR+U6-iRenilla (control), and incubated with 5 μg/mL puromycin (Wako, Osaka, Japan). Then, we selected puromycin-resistant clones. Parental PancO2 cells, VEGF-WT, and VEGF-KD clones were grown in 6-well plates and the VEGF content in the culture supernatant was measured in triplicates. As shown in Figure 1B, parental cells produced VEGF 3 340±151 pg/mL for 2×105 cells/24 h vs 3 102±285 pg/mL in VEGF-WT controls. VEGF production was significantly reduced in the pcPUR+U6-VEGFi1 stable clones. VEGF-KD7 produced VEGF (152±16 pg/mL) and was used in subsequent experiments.
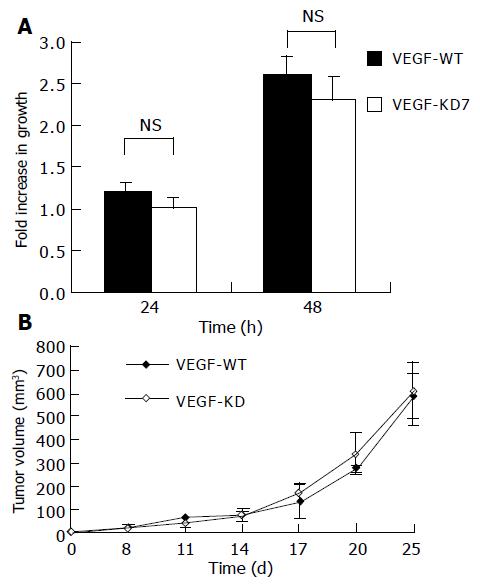
To investigate the effect of VEGF knockdown on the cell proliferation potential in vitro, 3×104 VEGF-KD7 and VEGF-WT cells were grown in 24-well plates. The numbers of viable cells were assessed using the MTT assay. As shown in Figure 2A, there was no statistical difference in cell proliferation between these two cell lines. To study the cell growth in vivo, 2×106 VEGF-KD7 or VEGF-WT cells were injected into C57BL/6 mice subcutaneously. Small tumors were typically observed, 1 wk after tumor implantation, measured, and the volume was calculated as [(length (mm) ×width (mm)2)]/2. As shown in Figure 2B, the growth of the VEGF knockdown cell line and control cells was not statistically different.
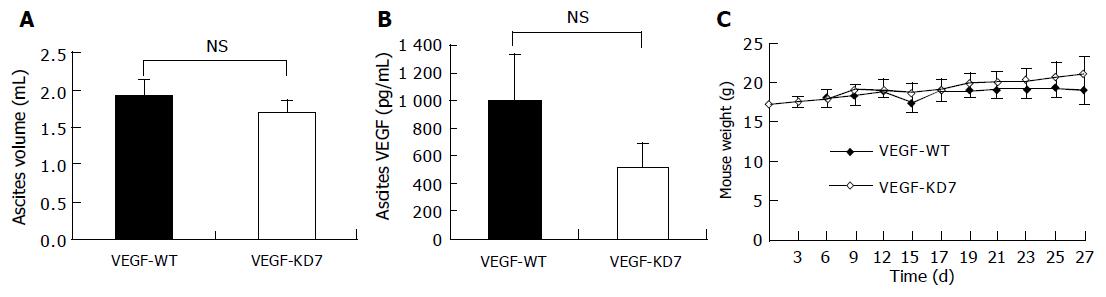
Ascites was induced by the intraperitoneal injection of 1×106 VEGF-KD7 or VEGF-WT cells into C57BL/6 mice. Ascites accumulation and peritoneal dissemination occurred about 2 wk after cancer cell inoculation. The ascites was bloody in both groups. As shown in Figure 3A, the volume of ascites did not differ between the VEGF-KD7 cell line and control VEGF-WT cells (1.9±0.22 vs 1.7±0.16 mL, P = 0.10). Next, we measured the concentration of VEGF in ascites. As shown in Figure 3B, the VEGF tended to be lower in the VEGF-KD7 cell line (517±181) compared with VEGF-WT cells (1 001±331), but the difference was not significant (P = 0.054). Both study groups showed extensive macroscopic peritoneal dissemination, but there was no difference in tumor weight between VEGF-KD7 and VEGF-WT cells (0.63±0.15 g vs 0.56±0.16 g, P = 0.8). To analyze the systemic effect, the mice were weighed every 2 or 3 d. As shown in Figure 3C, no difference was observed between VEGF-KD7 and VEGF-WT cells. The survival of mice inoculated with VEGF-KD7 or VEGF-WT cells did not differ statistically (35±4.7 d vs 38.2±4.2 d, P = 0.29).
Pancreatic cancer progression is associated with the accumulation of ascites in the peritoneal cavity. At least three different pathological events cause ascites, i.e., reduced lymphatic drainage from the peritoneal cavity: the obstruction of lymphatic vessels by tumor cells; hyperpermeability of the microvessels lining the peritoneal cavity; and angiogenesis[17]. VEGF contributes to ascites formation by enhancing vascular permeability and promoting new vessel growth[4].
To investigate the role of VEGF secreted from cancer cells in ascites formation following pancreatic cancer progression, we established VEGF knockdown pancreatic cancer cell lines using the stable RNAi method. The stable RNAi system is a useful tool for analyzing endogenous gene silencing[11-13]. Using this system, we were the first to succeed in establishing a VEGF knockdown stable cell line, and to analyze its effect in vivo. Others have used VEGF knockdown cells, but their analyses involved in vitro experiments[18].
Recent studies using fibrosarcomas generated from VEGF-null RAS-transformed mouse embryonic fibroblasts concluded that VEGF production in stromal compartments plays a critical role in tumor angiogenesis[10]. It has also been reported that tumor-infiltrating lymphocytes produce a number of potent angiogenic growth factors, cytokines, and proteases[19]. In another study, we used mouse syngenic colon cancer cells, which do not express VEGF protein in vitro, but the levels of VEGF protein expression in the tumor tissues did not differ statistically from PancO2 tumors (data not shown). Although VEGF protein expression was significantly reduced in our VEGF-KD7 cell line in vitro, the VEGF concentration in the ascites fluid was not statistically different compared with VEGF-WT cells. During tumor progression, infiltrating cells to the stroma produce a number of potent angiogenic growth factors, cytokines, and proteases. These findings indicate that cancerous compartments, such as the stromal tissues of a tumor, can play a critical role as the scaffold in VEGF production, and VEGF production in tumor tissues is not solely dependent on tumor cells. The accumulation of tumor ascites maybe induced by VEGF-dependent increase of permeability in the blood vessels of the peritoneum, and stromal compartments of the tumor tissue and peritoneum plays a critical role in VEGF-mediated ascites formation.
VEGF is a key mediator of tumor angiogenesis. Recent reports indicate that blocking VEGF is an effective strategy for treating human cancer[20]. The VEGF-specific antibody bevacizumab has anti-vascular effects in human rectal cancer[21]. Malignant ascites formation might be mediated in a cancer cell-type independent manner, and anti-VEGF strategy could be applied to various tumors regardless of whether they secrete VEGF.
We thank Mitsuko Tsubouchi for technical assistance.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Yeo KT, Wang HH, Nagy JA, Sioussat TM, Ledbetter SR, Hoogewerf AJ, Zhou Y, Masse EM, Senger DR, Dvorak HF. Vascular permeability factor (vascular endothelial growth factor) in guinea pig and human tumor and inflammatory effusions. Cancer Res. 1993;53:2912-2918. [PubMed] |
| 2. | Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2734] [Cited by in RCA: 2678] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 3. | Akutagawa N, Nishikawa A, Iwasaki M, Fujimoto T, Teramoto M, Kitajima Y, Endo T, Shibuya M, Kudo R. Expression of vascular endothelial growth factor and E-cadherin in human ovarian cancer: association with ascites fluid accumulation and peritoneal dissemination in mouse ascites model. Jpn J Cancer Res. 2002;93:644-651. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029-1039. [PubMed] |
| 5. | Berse B, Brown LF, Van de Water L, Dvorak HF, Senger DR. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell. 1992;3:211-220. [RCA] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 667] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 6. | Hlatky L, Tsionou C, Hahnfeldt P, Coleman CN. Mammary fibroblasts may influence breast tumor angiogenesis via hypoxia-induced vascular endothelial growth factor up-regulation and protein expression. Cancer Res. 1994;54:6083-6086. [PubMed] |
| 7. | Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192:150-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Pilch H, Schlenger K, Steiner E, Brockerhoff P, Knapstein P, Vaupel P. Hypoxia-stimulated expression of angiogenic growth factors in cervical cancer cells and cervical cancer-derived fibroblasts. Int J Gynecol Cancer. 2001;11:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Viloria-Petit A, Miquerol L, Yu JL, Gertsenstein M, Sheehan C, May L, Henkin J, Lobe C, Nagy A, Kerbel RS. Contrasting effects of VEGF gene disruption in embryonic stem cell-derived versus oncogene-induced tumors. EMBO J. 2003;22:4091-4102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Dong J, Grunstein J, Tejada M, Peale F, Frantz G, Liang WC, Bai W, Yu L, Kowalski J, Liang X. VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J. 2004;23:2800-2810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 225] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 11. | Brummelkamp TR, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002;2:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 821] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 12. | Miyagishi M, Taira K. U6 promoter-driven siRNAs with four uridine 3' overhangs efficiently suppress targeted gene expression in mammalian cells. Nat Biotechnol. 2002;20:497-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 526] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 13. | Imamura T, Kanai F, Kawakami T, Amarsanaa J, Ijichi H, Hoshida Y, Tanaka Y, Ikenoue T, Tateishi K, Kawabe T. Proteomic analysis of the TGF-beta signaling pathway in pancreatic carcinoma cells using stable RNA interference to silence Smad4 expression. Biochem Biophys Res Commun. 2004;318:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Morikane K, Tempero RM, Sivinski CL, Nomoto M, Van Lith ML, Muto T, Hollingsworth MA. Organ-specific pancreatic tumor growth properties and tumor immunity. Cancer Immunol Immunother. 1999;47:287-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M, Hisaminato A, Fujiwara T, Ito Y, Cantley LC. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19:6778-6791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 612] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 16. | Kanai F, Kawakami T, Hamada H, Sadata A, Yoshida Y, Tanaka T, Ohashi M, Tateishi K, Shiratori Y, Omata M. Adenovirus-mediated transduction of Escherichia coli uracil phosphoribosyltransferase gene sensitizes cancer cells to low concentrations of 5-fluorouracil. Cancer Res. 1998;58:1946-1951. [PubMed] |
| 17. | Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G, Giavazzi R. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 2003;63:5224-5229. [PubMed] |
| 18. | Zhang L, Yang N, Mohamed-Hadley A, Rubin SC, Coukos G. Vector-based RNAi, a novel tool for isoform-specific knock-down of VEGF and anti-angiogenesis gene therapy of cancer. Biochem Biophys Res Commun. 2003;303:1169-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11271] [Article Influence: 490.0] [Reference Citation Analysis (2)] |
| 20. | Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1788] [Cited by in RCA: 1891] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 21. | Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1468] [Cited by in RCA: 1442] [Article Influence: 68.7] [Reference Citation Analysis (0)] |