INTRODUCTION
Churg and Straus[1] histopathologically investigated a group of 13 patients who first developed asthma, followed by fever, hypereosinophilia and systemic small vessel vasculitis noted that it was characterized by necrotizing vasculitis and extravascular granuloma with hypereosinophilia, which distinguished it from classic PAN. The following year, Zeek[2,3] referred to this vasculitis as allergic granulomatous angitis and distinguished it from hypersensitivity vasculitis as a type of necrotizing vasculitis. We have therefore referred to this as Churg-Strauss syndrome, a disease which is manifested as asthma, eosinophilia and vasculitis. When its biopsy specimen histopathologically shows hypereosinophilia or extravascular granuloma, we refer to it as allergic granulomatous angitis. Mononeuritis[4,5] multiplex and gastrointestinal involvement are common in CSS, but cholecystitis is less common. In the literature, six cases[6-11] of cholecystitis complicated with CSS have been reported, while for liver abscesses, only one[14] has been reported. We describe herein a case of CSS presented with colon erosion, cholecystitis and liver abscesses.
CASE REPORT
A 21-year-old woman was admitted on July 2, 2002, with a complaint of abdominal pain. The patient had a history of bronchial asthma and sinusitis 3 years prior to admission, and mentioned that her mother was an asthmatic. A year prior to admission, the patient had been diagnosed with eosinophilic infiltrating pneumonia and was treated with an oral corticosteroid, which had been tapered and continued until admission. On June 22, 2002 she felt severe pain in her left upper quadrant subsequently and on June 27, she consulted a doctor at our hospital and had her stomach examined. However, gastrofiberscopy showed no abnormalities. The patient was suspected to be constipated and thus administered a laxative. However, her abdominal pain had persisted, and so she went to the emergency department and was admitted for further evaluation and treatment. On physical examination, no rash or lymphadenopathy was found, no crackle was present over both lung fields, and no cardiac murmurs were heard, her abdomen was soft and flat, and there was no tenderness. In addition, her lower legs were not edematous. On neurologic examination, the patient was alert and oriented. Her cranial nerve functions, muscle strength, and deep-tendon reflexes were normal. Her hematologic findings showed a leukocyte count of 2.93×104 per microliter with 56.0% eosinophils, and a platelet count of 39.9×104 per microliter. Her C-reactive protein level was 2.5 mg/dL, but her lactate dehydrogenase was elevated to 398 IU/L. Her total protein was 7.7 g/dL with γ-globulin at 25.2%, serum alanine aminotransferase (ALT) level was 118 IU/L (normal range of 10-35 IU/L), aspartate aminotransferase (AST) level was 63 IU/L (normal range of 10-35 IU/L), and alkaline phosphatase level was 254 IU/L (normal range of 120-340 IU/L). In addition, the patient’s and Leucine aminopeptidase level was 51 IU/L (normal range of 23-50 IU/L), and her γ-glutamyl-transpeptidase level was 43 IU/L (normal range of 8-60 IU/L). Her total bilirubin and albumin levels were within normal limits and her immunoglobulin IgG and IgE levels were 1 980 mg/dL and 27 U/mL, respectively. A test for antinuclear antibody was borderline positive, rheumatoid factor was positive, but C-anti-neutrophil cytoplasmic antibodies (C-ANCA) and myeloperoxidase anti-neutrophil cytoplasmic antibodies (MPO-ANCA) were negative. The circulating levels immune complex CH50, C3 and C4 levels in the serum were all normal, renal function was almost normal, and there was no hematuria or sedimentation in the urine. Sputum bacteriology showed only normal flora and no tubercle bacilli and a worm egg test was negative, but the results of an occult blood test were positive. Radiographs of the chest showed a faintly nodular density in the left lower lung field, but radiographs of the abdomen were almost normal. A CT showed that her gallbladder had shrunk, but her liver and pancreas, GI tract and colon were normal. Ultrasonography (US) showed similar findings. Spontaneous pain emanating from her epigastrium to her left upper quadrant was so strong that the repeated use of analgesics had no effect. A colonoscopy showed multiple aphthous ulcers and erosion from the rectum to the ileocecal region (Figure 1). Furthermore, a colonic biopsy specimen showed marked eosinophilic infiltration from the muscular layer of the mucosa to the submucosa, and for the small vessels, lumen stenosis with thickening of the intima indicative of vasculitis was noted (Figure 2). On the basis of her clinical features such as bronchial asthma preceding vasculitis, the laboratory findings including remarkable leukocytosis and eosinophilia, and histopathological findings of eosinophilic vasculitis, a diagnosis of CSS was established. She was administered with oral prednisolone daily since her 10th d in hospital, so her eosinophil count gradually decreased and abdominal pain slowly disappeared. However, she was examined by abdominal CT because she developed a low grade fever, and laboratory findings showed increased liver function on the 15th d. CT showed that the gallbladder was distended and had a thickened wall (Figure 3). We could not detect stones in her gallbladder, but found low density areas, that were faintly enhanced at S6 and S8 of right lobe of the liver ,which we suspected to be abscesses (Figure 3). US showed similar findings. From these examinations, our diagnosis was cholecystitis and liver abscesses, and so intravenous antibiotic (IPM/CS) were administered. CT on the 38th d in hospital revealed low density areas at S6 and S8 that were fading, and that her gallbladder was becoming atrophic with simultaneous wall thickening. After 41 d in hospital, her prednisolone was gradually tapered. She continued to be asymptomatic without recurrence, and was discharged, on the 58th d. After discharge, US revealed a projection in her gallbladder 20 mm in diameter. We proposed its surgical removal because we could not distinguish it from a polyp. Therefore, a laparoscopic cholecystectomy was performed on October 15. No polyp was found, but debris was noted in the resected specimen. Histopathological findings of the resected gallbladder revealed necrotized vessels with occlusive intimal proliferation below the tunica serosa vesicae felleae, which had passed the peak of fibrinoid necrosis. We could not detect eosinophilic infiltration in the specimen (Figure 4), and a liver biopsy performed at the same time showed no remarkable abnormalities.
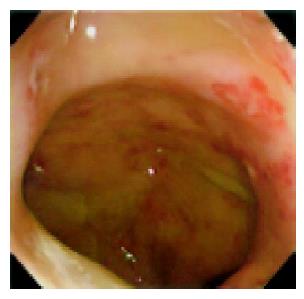
Figure 1 Colonoscopy showing multiple aphthous ulcers and erosion from the rectum to the ileocecal region.
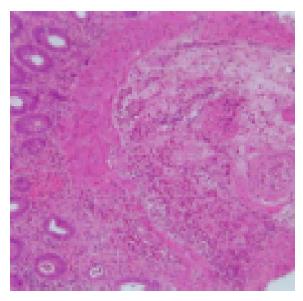
Figure 2 A colonic biopsy specimen showing marked cellular infiltration from the muscular layer of the mucosa to the submucosa and small vessels, lumen stenosis with thickening of the intima, indicative of vasculitis.
A H&E,×100.
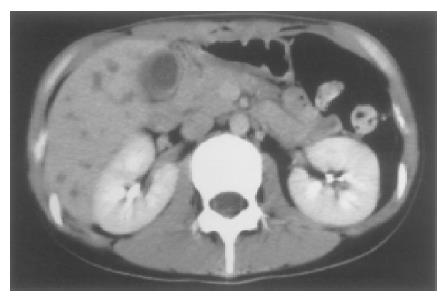
Figure 3 CT showing the gallbladder distended with a thickened wall.
In addition, low density areas faintly enhanced at S6 and S8 in the right lobe of the liver suspected to be abscesses are seen.
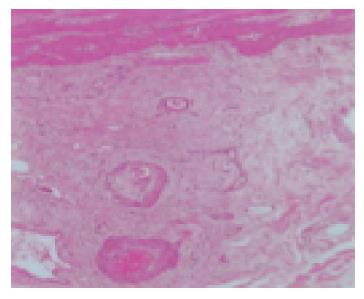
Figure 4 Histopathological findings of the resected gallbladder revealed necrotized vessels with occlusive intimal proliferation below the tunica serosa vesicae felleae, which had passed acute phase in the resected specimen.
We could not detect eosinophilic infiltration in this specimen.
DISCUSSION
Six criteria used for the classification of CSS were selected: (1) Bronchial asthma;( 2) Eosinophilia >10% by differential WBC count; (3) Mononeuropathy or polyneuropathy, (4) Non-fixed pulmonary infiltrates on roentgenography, (5) Paranasal sinus abnormality, and (6) Biopsy containing a blood vessel with extravascular eosinophils[6,12]. Masi et al reported that the existence of four or more of these six criteria yields a sensitivity of 85% and a specificity of 99.7%[12]. The present patient did not suffer from mononeuropathy or polyneuropathy, but fulfilled the other five criteria. Furthermore, she suffered from leukocytosis, an elevated RF level, thrombocytosis except an increased IgE level, and her histopathological findings showed necrosis with marked eosinophilic infiltration. Therefore, we diagnosed her condition as CSS. We can cite as differential diagnosis, eosinophilic leukocytosis, Wegener’s granulomatosis, microscopic polyangitis, and PAN (polyarteritis nodosa). CSS is similar to eosinophilic leukocytosis in clinical manifestation, but we could distinguish it from the condition of this case as eosinophilic leukocytosis in thrombocytopenia was not observed and so many impaired internal organs were noted. We could distinguish this case from Wegener’s granulomatosis in respect that she did not suffer from kidney trouble or exhibit positive reaction by the C-ANCA[13] test, supported by her histopathological findings. In addition, her past history of sinusitis was typical of one of the major features of CSS in America. She was not afflicted with microscopic polyangitis, because she did not exhibit any positive reaction for MPO-ANCA or the rapid progression of glomerulonephritis, and she did not have PAN because she did not have the various symptoms and showed remarkable eosinophilic infiltration as seen from her histopathological findings. We could only diagnose her with typical CSS judging from her initial course of asthma followed by systemic small vessel vasculitis after 4 years, and because she also had been suffering from eosinophilic infiltrating pneumonia before admission. Lanham et al., reported that for about 40% of case of CSS, such pneumonia with eosinophilic infiltration proceeds systemic small vessel vasculitis[5]. The present case developed cholecystitis and liver abscesses, and we could not detect stones in the resected GB. Therefore, we diagnosed her as having acalculous cholecystitis. There was no eosinophilic infiltration found in the resected specimen of the gallbladder because steroid treatment had been carried out. However, we detected necrotized vessels that had passed acute phase in that specimen. Therefore, we believed that her cholecystitis had been caused by CSS. Regarding her liver abscesses, since we could detect no remarkable abnormalities such as those found in the gallbladder among the pathological findings of her liver, we thought that the abscesses had been caused by infection via the blood circulation from cholecystitis or colitis. We report this case because the clinical manifestations of CSS usually include gastroenteritis and ischemic ulcerations of the stomach as well as the small and large bowel, but less often include cholecystitis and liver abscesses.