Published online Aug 28, 2005. doi: 10.3748/wjg.v11.i32.5075
Revised: April 5, 2005
Accepted: April 9, 2005
Published online: August 28, 2005
Chronic pancreatitis is a relatively common disease. We encountered two different cases of belatedly demonstrated pancreatic carcinoma featuring underlying chronic pancreatitis. The first case was one that was highly suspected as that of a malignancy based upon imaging study, but unfortunately, it could not be confirmed by intra-operative cytology at that time. Following this, the surgeon elected to perform only conservative bypass surgery for obstructive biliary complication. Peritoneal carcinomatosis was later noted and the patient finally died. The second case, a malignant mucinous neoplasm, was falsely diagnosed as a pseudocyst, based upon the lesion’s sonographic appearance and associated elevated serum amylase levels. After suffering repeated hemoptysis, the patient was found to exhibit lung metastasis and peritoneal seeding. We reviewed some of the literature, including those studies discussing chronic pancreatitis predisposing to a malignant change. These two case analyses illustrate clearly that the diagnosis for such conditions, which is simply based upon imagery or pathological considerations may end up being one of a mistaken malignancy. Some of our suggestions for the treatment of such malignancies as revealed herein include, total pancreatomy for univocal mass lesion, and needle aspiration of lesion-contained tissue for amylase, CA199 and CEA levels for a suspicious cystic pancreatic mass.
- Citation: Leung TK, Lee CM, Wang FC, Chen HC, Wang HJ. Difficulty with diagnosis of malignant pancreatic neoplasms coexisting with chronic pancreatitis. World J Gastroenterol 2005; 11(32): 5075-5078
- URL: https://www.wjgnet.com/1007-9327/full/v11/i32/5075.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i32.5075
We herein report on two cases of pancreatic malignancy, an adenocarcinoma and a mucinous cystadenocarcinoma, both of which featured an underlying chronic pancreatitis. The first case was incorrectly diagnosed initially, even though such diagnosis followed multiple-modality imagery studies and intra-operative frozen-section pathology. The second case was, initially, falsely diagnosed as a case of a pseudocyst that had missed the ‘golden period’ of opportunity for surgical treatment for this relatively non-aggressive malignant tumor. The true diagnosis for this case was made subsequent to the discovery of the widespread distribution of mucinous tumor cells to the lungs and peritoneum. In an attempt to improve our image-interpretation skills, and to provide some more-effective suggestions for clinicians as regards to the appropriate diagnostic procedures for future cases of a similar nature, we have discussed these cases and reviewed some of the related literature.
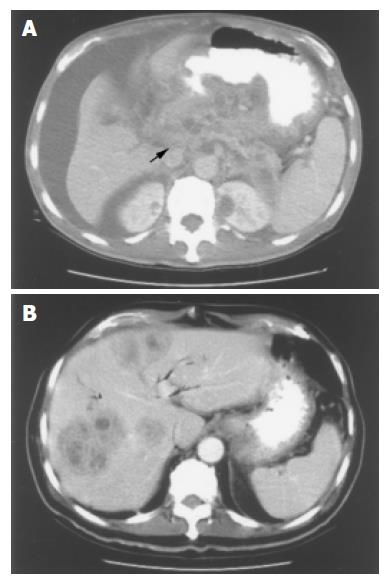
A 60-year-old female patient presented at our hospital, suffering from diabetes mellitus and chronic pancreatitis, and revealed a smoking habit of greater than 30 years. On one previous occasion, this patient was admitted to a local hospital on a complaint of chronic inflammation complicating post-inflammatory obstructive jaundice. Two years subsequent to this event, this patient suffered a relapse; she complained of abdominal fullness and poor appetite. Tea-colored urine and yellowish-colored skin were both noted, the patient was diagnosed with biliary obstruction, and her condition failed to improve following admission. The patient was then referred to our OPD for further evaluation, at which time her condition had progressed to one of general malaise and notable body-weight loss. Serum amylase, CA-199 and CEA levels were, respectively, 58 IU/L (normal <100), 1 387 U/mL (normal <37) and 5.75 ng/mL (normal <4.6). Endoscopic retrograde cholangiopancreatography (ERCP) was performed, but obviously not sufficiently and effectively, it only revealed pancreatic-duct dilatation and failed to provide appropriate visualization of the common bile duct (CBD). Subsequent enhanced CT and MRCP studies revealed dilatation of the CBD, IHD, and MPD, and an ill-defined, heterogeneous enhanced mass located in the uncinate process of the pancreatic head (Figure 1A). Our impression at that time was malignant pancreatic lesion complicating CBD obstruction. Surgical intervention was then conducted, it revealed the presence of a large, stony hard mass, measuring 5-6 cm in diameter, located at the pancreatic head and being tightly adherent to the portal vein and the common hepatic artery. Further, we noted a 1.5 cm-diameter lymph node located along the superior mesenteric artery. A gall-bladder stone was also noted. Intra-operative frozen-sectioning of a needle biopsy from the pancreatic head and body revealed fibrosis and chronic inflammation with lymphocytic infiltration into the fibrotic stroma. Intralobular and perilobular fibrosis with atrophy of acini admixed with disorderly arranged islets were also seen, but no evidence of malignancy was apparent, a result that contrasted our initial impression. The patient then underwent a bypass surgery including cholecystectomy, choledochojejunostomy with Roux-en-Y and gastrojejunostomy. Two months later, this patient again presented at our hospital with similar symptoms to those detected at her previous admission, computed tomography (CT) revealing multiple heterogenous ill-defined hepatic tumors (Figure 1B). At this time, we strongly suspected metastatic lesions according to our past impressions of her condition, the past history of this patient, and the updated CT findings. Sonographically guided biopsy was conducted, but the subsequent histopathological report concluded that the lesion was a liver abscess. The patient was administered antibiotics and other supportive treatment. Her condition improved and remained stable for about 2 mo, following which, her condition deteriorated, and she was admitted again to our institution due to progressive abdominal distension, poor appetite and further body-weight loss. The patient’s serum CA-199 level had elevated to 4 000 U/mL (normal <37). An abdominal CT scan was performed; it revealed a progressively enlarging heterogenous enhanced mass, located at the pancreatic body and tail section. This pancreatic mass had also invaded the lesser sac of the stomach, there being direct invasion into the stomach. The pancreatic mass had also resulted in celiac-trunk encasement and the encirclement of the superior mesenteric vessels, with ascites being massive. A condition of pancreatitis superimposed onto malignant change and metastatic peritoneal seeding into the transverse mesocolon and sigmoid mesocolon was diagnosed. Pathological review finally confirmed intraperitoneal carcinomatosis due to the presence of an advanced pancreatic carcinoma, the patient expired about 5 mo later.
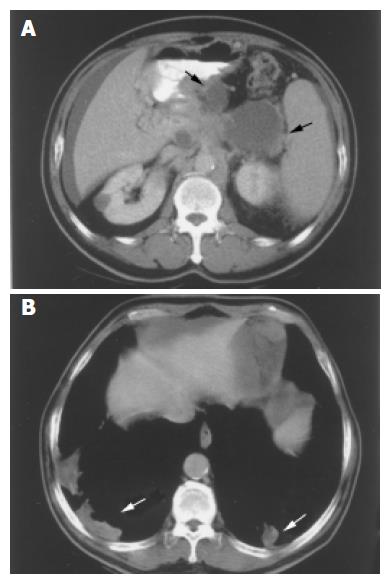
This 62-year-old male patient featured chronic alcoholism over a period of more than 30 years. Chronic pancreatitis and liver cirrhosis were diagnosed for this patient and he was recommended regular OPD follow up. Slightly elevated serum amylase levels were recorded for this patient, and for approximately 3 mo, he suffered from intermittent abdominal fullness; diffuse dull pain and a general sensation of nausea. Sonographic evaluation revealed multiple pancreatic cystic lesions, ascites, splenomegaly and portal hypertension, with no evidence of tumor septation, loculation, no cystic-wall calcification, and no evidence of the presence of any soft-tissue masses. The initial diagnosis for this patient was chronic pancreatitis with associated pseudocyst formation, based upon the combined results of laboratory and sonographic investigations. Three months later, this patient’s condition had, by-and-large, persisted, and upon presentation at our hospital for further evaluation, he was found to be suffering from hemoptysis. This patient’s serum amylase, CA199 and CEA levels were reported as, respectively, 150 IU/L (normal <100), 3 841 U/mL (normal <37) and 9.79 ng/mL (normal <4.6). A chest X ray revealed hazy bilateral shadows over the lower lung fields. The patient underwent CT investigation, which revealed multiple cystic pancreatic masses (Figure 2A) confluent with contrast-enhanced soft-tissue components, the largest two masses measuring greater than 7.7 and 3.7 cm in diameter, these lesions arising from the pancreatic body and tail. Fluid distribution within the intraperitoneal and pelvic cavities was described, respectively, as extensive and moderate. CT investigation also revealed multiple foci of alveolar patches (Figure 2B) with a mean density of 10-15 HU. PET scanning was then performed, the results demon-strating multiple areas of FDG uptake including the posterior and bilateral aspects of the lower lungs, the middle-upper abdomen (pancreatic body featured irregular shapes) and the middle-lower abdomen. CT guided fine needle aspiration of the para-pancreatic cystic fluid [f] revealed normal level of amylase, but enormous elevation of CA-199 (32 000 001 U/mL) and CEA (43 000 ng/mL). CT guide biopsy for the lung patch also revealed mucinous adenocarcinoma metastasis. Combined with the results of images and the histopathological investigations, the presence of pancreatic mucinous adenocarcinoma complicated by lung and peritoneal seedings was confirmed.
Chronic pancreatitis may eventuate as a consequence of recurrent acute inflammation in the pancreas, such a condition often affecting neighboring structures and their functions. Typical image manifestations of chronic pancreatitis include pancreatic calcification on KUB, atrophic changes and/or the presence of coexistent focal hypertrophy, pseudocyst formation and a dilated pancreatic duct as detected by sonographic or CT investigation. ERCP may be used to demonstrate abnormalities of the main pancreatic duct as also secondary branches. Other possible imagery findings for chronic pancreatitis include fatty replacement, fibrotic changes complicating duodenal stenosis with outflow obstruction and long tapered stenoses of the distal common bile duct with biliary obstruction, aneurysms or pseudoaneurysms of the splanchnic arteries and splenic-vein thrombosis. The predisposing factors for pancreatic adenocarcinoma include chronic pancreatitis, diabetes mellitus, smoking and a potential underlying genetic risk[1]. The pathophysiological and molecular events underlying chronic pancreatitis have been reported to predispose to the development of, or potentiate the growth of, pancreatic adenocarcinomas[2]. Mutation of the codon 12 K-ras gene, tumor angiogenesis and microvascular proliferation are, reportedly, genetically-linked aberrations[2] commonly associated with the development of pancreatic adenocarcinoma.
Discrimination between a well-differentiated pancreatic carcinoma[3] and chronic fibrotizing pancreatitis is a challenge not only to the radiologist[4], but also to the pathologist[5,6]. As mentioned above, pancreatic adenocarcinoma and chronic pancreatitis may coexist, and a malignant tumor can develop as a complication of long-standing chronic pancreatitis. On the other hand, pancreatic carcinoma is frequently accompanied by chronic inflammation. The majority of adenocarcinomas induce a striking desmoplastic stromal reaction within the pancreas, which may effectively mimic the macroscopic view of vigorous fibroblast proliferation. Microscopically, pancreatitis can be mistaken for pancreatic carcinoma, because the actively growing connective tissue associated with pancreatitis, tends to irregularly separate the existing pancreatic ducts. This continuous regenerative activity may lead to regressive atypia that resembles malignancy[5]. A retrospective study of 5 837 previously histopathologically confirmed pancreatic carcinoma patients, reported little more than 0.5% false-positive results[7]. In our opinion, for the first case described above, only total pancreatomy with tissue sent for a precise histopathological examination, instead of frozen-section biopsy, could have increased the likelihood of arriving at a correct diagnosis.
Pseudocyst formation is present for about 30% of chronic pancreatitis cases, such lesions being a collection of pancreatic “juice” enclosed by a wall of fibrous or granulation tissue. Pseudocyst lesions of the pancreas tend to populate the majority of pancreatic cystic lesions. For our second case, however, the diagnostic challenge was to differentiate correctly between a cystic neoplasm and pseudocysts for the patient featuring underlying chronic pancreatitis. Patients who are afflicted with pseudocysts often reveal a history of acute or chronic pancreatitis, whilst most of those featuring cystic tumors lack such antecedent factors. The typical findings of a pseudocyst upon a CT scan, include the presence of a well-defined, non-epithelial, fibrous wall around the cyst lesion. Pseudocysts are typically round or ovoid in configuration and, apart from the assessment of pseudocysts by CT, pseudocysts can generally be identified by hypovascularity upon angiography; and the detection of a communication between the cyst and the pancreatic ductal system upon ERCP. On the other hand, MRI has its advantages over CT in the evaluation of pancreatic pseudocysts. T1W MRI with gadolinium could be more sensitive to demonstrate internal structure of cyst-like lesion, such as very thin septa, which may not be detected by enhanced CT scan. MRCP could help for ruling out of pseudocysts by determining whether they communicate with the pancreatic duct. Cystic neoplasm of the pancreas would appear to be rare, it constituting less than 4% of all pancreatic neoplasms[8]. The features of a cystic neoplasm, as compared to a pseudocyst, include, for the former, the presence of septa, loculation, solid components and/or cystic-wall calcification. It would appear that sonography is not sufficiently reliable to unequivocally distinguish between pseudocysts and other cystic neoplasms of the pancreas. In our opinion, the early scheduling of abdominal CT subsequent to sonography having been completed for our second case, probably would have hastened the correct diagnosis for this patient. Moreover, analysis of cyst-contained fluid[9,10], can also aid in the evaluation of cystic neoplasms, in that elevated fluid amylase levels are characteristic of over 95% of pseudocysts, and a normal serum amylase level can be used to exclude the presence of a pseudocyst. Fluid CEA levels can become elevated for a variety of cystic lesions including mucinous cystic neoplasm[11], intra-ductal papillary mucinous tumors and some pseudocysts, but such levels are always low or normal for cases of serious cystadenoma. A summary of the laboratory-elicited features of cystic fluid for the purposes of distinguishing between pancreatic cysts and the most-common type of cystic tumors is presented in Table 1.
| Pseudocyst | Serous cystadenoma | Mucinous cystadenoma | Mucinous cystadenocarcinoma | |
| Amylase (normal <100 IU/L) | High | Low | Low | Low |
| CA 199 (normal<37 U/mL) | Low | Low | Intermediate | High |
| CEA (normal<4.6 ng/mL) | Low | Low | High | High |
In conclusion, if an accurate diagnosis of suspicious pancreatic lesions for patients suffering from chronic pancreatitis is not able to be reached based upon appropriate imagery studies, we suggest fine-needle aspiration biopsy[9] of cystic neoplasms for subsequent laboratory investigation, or, alternatively, wide resection of the pancreatic mass for a precise histopathological evaluation, rather than simple observation[12].
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Hall Pde L, Wilentz RE, de Klerk W, Bornman PP. Premalignant conditions of the pancreas. Pathology. 2002;34:504-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Banerjee SK, Zoubine MN, Mullick M, Weston AP, Cherian R, Campbell DR. Tumor angiogenesis in chronic pancreatitis and pancreatic adenocarcinoma: impact of K-ras mutations. Pancreas. 2000;20:248-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Machado MC, Montagnini AL, Machado MA, Falzoni R, Volpe P, Jukemura J, Abdo EE, Penteado S, Bacchella T, Monteiro-Cunha JE. Cystic neoplasm diagnosed as pancreatic pseudocyst: report of 5 cases and review of the literature. Rev Hosp Clin Fac Med Sao Paulo. 1994;49:246-249. [PubMed] |
| 4. | Civello IM, Frontera D, Viola G, Maria G, Crucitti F. Cystic neoplasm mistaken for pancreatic pseudocyst. Hepatogastroenterology. 1996;43:967-970. [PubMed] |
| 5. | Zalatnai A. Pathologic diagnosis of pancreatic cancer--facts, pitfalls, challenges. Orv Hetil. 2001;142:1885-1890. [PubMed] |
| 6. | Nieman JL, Holmes FF. Accuracy of diagnosis of pancreatic cancer decreases with increasing age. J Am Geriatr Soc. 1989;37:97-100. [PubMed] |
| 7. | Alanen KA, Joensuu H. Long-term survival after pancreatic adenocarcinoma--often a misdiagnosis? Br J Cancer. 1993;68:1004-1005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Bradley EL, Clements JL, Gonzalez AC. The natural history of pancreatic pseudocysts: a unified concept of management. Am J Surg. 1979;137:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 253] [Article Influence: 5.5] [Reference Citation Analysis (2)] |
| 9. | Pinto MM, Kaye AD. Fine needle aspiration of cystic liver lesions. Cytologic examination and carcinoembryonic antigen assay of cyst contents. Acta Cytol. 1989;33:852-856. [PubMed] |
| 10. | Safi F, Schlosser W, Falkenreck S, Beger HG. Prognostic value of CA 19-9 serum course in pancreatic cancer. Hepatogastroenterology. 1998;45:253-259. [PubMed] |
| 11. | Buetow PC, Rao P, Thompson LD. From the Archives of the AFIP. Mucinous cystic neoplasms of the pancreas: radiologic-pathologic correlation. Radiographics. 1998;18:433-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 85] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Scott J, Martin I, Redhead D, Hammond P, Garden OJ. Mucinous cystic neoplasms of the pancreas: imaging features and diagnostic difficulties. Clin Radiol. 2000;55:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 64] [Article Influence: 2.6] [Reference Citation Analysis (0)] |