Published online Aug 21, 2005. doi: 10.3748/wjg.v11.i31.4899
Revised: February 13, 2005
Accepted: February 18, 2005
Published online: August 21, 2005
AIM: To construct the expression vector of B3 (scdsFv)-SEA (D227A) and to identify its binding and cytotoxic ability to B3 antigen positive carcinoma cell lines.
METHODS: This fusion protein was produced by a bacterial expression system in this study. It was expressed mainly in the inclusion body. The gene product was solubilized by guanidine hydrochloride, refolded by conventional dilution method, and purified using SP-sepharose cation chromatography.
RESULTS: The expression vector B3 (scdsFv)-SEA-PET was constructed, the expression product existed mainly in the inclusion body, the refolding product retained the binding ability of the single-chain antibody and had cytotoxic effect on HT-29 colon carcinoma cells. The stability assay showed that the resulting protein was stable at 37 °C.
CONCLUSION: This genetically engineered B3 (scdsFv)-SEA fusion protein has bifunction of tumor targeting and tumor cell killing and shows its promises as an effective reagent for tumor-targeted immunotherapy.
- Citation: Wang JL, Zheng YL, Ma R, Wang BL, Guo AG, Jiang YQ. Disulfide-stabilized single-chain antibody-targeted superantigen: Construction of a prokaryotic expression system and its functional analysis. World J Gastroenterol 2005; 11(31): 4899-4903
- URL: https://www.wjgnet.com/1007-9327/full/v11/i31/4899.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i31.4899
Bacterial superantigen Staphylococcal enterotoxin A (SEA) is an efficient activator of cytotoxic T cells when presented on MHCII antigens and shows a potential ability in cancer immunotherapy[1-3]. But SEA also has cytotoxicity against the normal cells when used to kill carcinoma cells and has no effect on MHCII carcinoma cells. The monoclonal antibody (mAb) preparation technique has brought about a new method for cancer therapy known as tumor-targeting therapy. Which therapy can solve the two problems mentioned above? The mAb B3 is a murine antibody directed against a carbohydrate antigen in the LeY family, that is found on the surface of many mucinous carcinomas of the colon, stomach, ovaries, breast, and lung as well as some epidermal carcinomas. Because mAbB3 reacts with a limited number of normal tissues, it is an ideal candidate for the treatment and diagnosis of cancer[4]. Immunotoxins made from mAb B3 are cytotoxic to various human cancer cell lines that express B3 antigen on their surface, and some of them have been evaluated in clinical or preclinical trials[5].
Antibodies penetrate tumors slowly and take several days to mix completely within a tumor because of their large size[6]. Fv fragments of antibodies are heterodimers of the heavy chain variable domain (VH) and the light chain variable domain (VL), and the smallest functional modules of antibodies required for high affinity antigen binding. The polypeptide chains of whole IgG or Fab fragments are joined by a disulfide bond, but Fv fragments have no such inter-chain disulfide bridge and are therefore unstable[7]. Stable Fv fragments can be produced by making recombinant molecules in which the VH and VL domains are connected by a peptide linker (ScFv) or a disulfide bond (DsFv), so that the antigen binding site is regenerated in a single protein. However, many single-chain Fvs are unstable. When they are fused to toxins, the resulting recombinant immunotoxins are also unstable, presumably owing to aggregation during the transient separation of VH from VL by the peptide linker. DsFv is more stable than ScFv, but the low yield after renaturation limits its application[8]. A new type of engineered antibody, known as scdsFv has a linker and a disulfide bond between VH and VL, and is more stable than dsFv[8].
In this study, scdsFv of B3mAb (B3scdsFv) was fused to the SEA (D227A) by genetic engineering method and expressed in E.coli. The refolding protein has been proved to be stable at 37 °C. It can bind to tumor surface antigen and is cytotoxic to HT-29 colon cells, thus SEA can be used in tumor targeting therapy.
Template plasmids B3scFv-pET32a and SEA (D227A)-pET32a were constructed by our laboratory. Expression plasmid pET22b, E.coli TOP10, human colon carcinoma cell line HT-29, human hepatoma cell line SMMC-7721, human breast carcinoma cell line MCF-7 were all kept in our laboratory.
LATaqDNA polymerase and restricted enzymes were purchased from Takara Biotechnology Co., Ltd (Dalian, China). T4 DNA ligase and non-radioactive cell proliferation assay kit were obtained from Promega (USA). AKTA FPLC and Hitrap-SP sepharose column were products of Amersham Biosciences (Switzerland). Primer synthesizing and DNA sequencing were completed by Bioasia Co., Ltd (Shanghai, China).
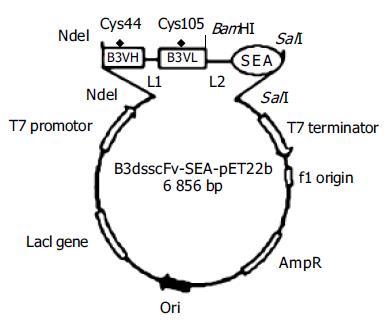
Mutated disulfide bond sites in B3VH (Cys44) and B3VL (Cys105) were designed as previously described[9,10]. A mutation in the linker between VH and VL was found in the template plasmid B3scdsFv-pET32a after DNA sequencing. Since the 6th base in GGGGSGGGGSGGGGS (linker) was mutated from G to S, we corrected the mutation by overlap PCR and ligated B3VH and B3VL at the same time. Four primers were designed according to the gene sequence in GenBank and the linker sequence (Table 1). Part of the linker sequence was involved in P2 and P3. Primers 1 and 2 were used for amplification of B3VH and primers 3 and 4 for B3VL. The purified B3VH and B3VL products were mixed at the ratio of 1:1 and then applied as the template for the amplification of B3scdsFv with primers 1 and 4. Then the purified B3scdsFv fragment was cloned into the DNA sequencing vector pMD-18T. The full length B3scdsFv was excised from pMD-18T-vector containing correct insert with NdeI and BamHI and subcloned into a similarly digested pET22b vector to produce the recombinant plasmid B3scdsFv-pET22b.
| Number | Long oligonucleotide sequences |
| 1 | 5’-GGC CAT ATG GAT GTG AAG CTG GTG-3’ |
| 2 | 5’-ACC TCC ACC TGA ACC GCC TCC ACC GGA GAC AGT GAC CAG-3’ |
| 3 | 5’-CTG GTC ACT GTC TCC GGT GGA GGC GGT TCA GGT GGA-3’ |
| 4 | 5’-CCG GGA TCC GCC CCC TTT AAT TTC CAG CTT TGT-3’ |
| 5 | 5’-ATTGGATCCGGAGGTTCAAGCGAGAAAAGCGAAGAAATA-3’ |
| 6 | 5’-CACGTCGACTTAACTTGTATATAAATATATAGCAATATGCATG-3’ |
The SEA gene was amplified by PCR using primer pair P5 and P6 and template SEA (D227A)-pET32a. The PCR products were purified and directly cloned into pMD-18T-vector and further cloned into pET22b (Figure 1).
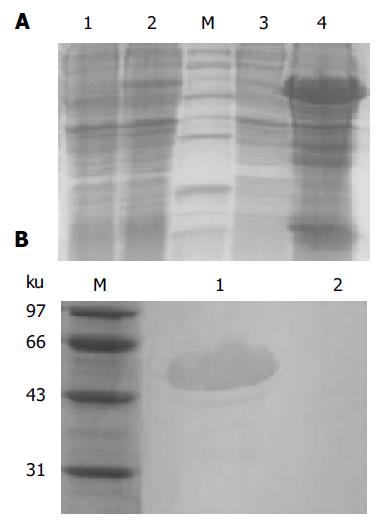
The bacterial strain BL21 (DE3) pLysS was used to express the fusion protein. Briefly, one positive clone was grown in LB medium containing 100 μg/mL ampicillin and induction was started by addition of IPTG (final concentration, 1 mmol/L) when the culture reached A600 = 0.5. After the culture was induced for 3 h at 37 °C, the cells were harvested. The bacterial pellet was resuspended in 50 mmol/L Tris-HCl, 5 mmol/L EDTA, pH 8.5, 0.8% NaCl, and sonicated, then centrifuged at 12 000 g for 15 min at 4 °C to separate the supernatant and the inclusion body, and analyzed on 15% SDS-PAGE.
Western blotting was performed to confirm B3scdsFv-SEA identity. Protein samples were dissolved by 15% SDS-PAGE under reducing conditions and using duplicate gels. One gel was Coomassie stained while the other was used for semi-dry electrophoretic transfer (Jim-X Biotechnology Co., Ltd, Dalian, China) of the proteins onto nitrocellulose membrane. The membrane was then blocked with 0.05 mol/L Tris-HCl, pH 7.4, 0.5 mol/L NaCl, 3% BSA and incubated with the rabbit polyclonal antibody against SEA for 1 h and followed by the incubation of HRP-coupled goat anti-rabbit IgG as secondary antibody. Finally, the recombinant B3scdsFv-SEA was visualized with Fast DAB peroxidase substrate. In this part, the B3scdsFv-SEA-pET22b before induction (total protein) was used as a negative control.
The inclusion body was washed twice in 0.05 mol/L Tris-HCl, pH 8.0, 20 mmol/L EDTA, 2% Triton X-100, 0.5 mol/L NaCl, then dissolved in 6 mol/L guanidine hydrochloride, 2 mmol/L EDTA, 0.05 mol/L Tris-HCl, pH 8.5, 10 mmol/L DTT and incubated for 3 h at room temperature. The denatured products were centrifuged for 10 min at 12 000 g at 4 °C. The supernatant was slowly diluted into the refolding solution containing 0.1 mol/L Tris-HCl, pH 8.0, 0.5 mol/L L-Arg, 2 mmol/L EDTA, 0.9 mmol/L oxidized glutathione at the ratio 1:30, refolded for 48 h at 10 °C.
The refolding solution was concentrated by hollow fiber cartridges, and buffer exchange was carried out at the same time. The denatured protein in 5 mmol/L phosphate buffer, pH 5.8 (buffer A) was loaded into a 5 mL sp-sepharose column pre-equilibrated by buffer A. The protein was eluted with a linear gradient from 0 to 0.5 mol/L NaCl in 0.04 mol/L phosphate buffer, pH 7.4 (buffer B). The eluted protein was analyzed on 15% SDS-PAGE.
Analysis for the binding of B3scdsFv-SEA to B3 positive tumor cells (including human colon carcinoma cell line HT-29, human hepatoma cell line SMMC-7721, human breast carcinoma cell line MCF-7) was performed as described previously[11]. Briefly, the prepared tumor cells were seeded in a 96-well plate containing 5103 cells per well. Cells were cultured for 48 h at 37 °C and the plate was washed thrice with PBS. After fixation with pre-cooled fixing solution (methanol/acetone solution, v/v = 1:1) for 30 min, the reaction was ended by addition of PBS containing 1% H2O2. Blocking was performed with 1% bovine serum albumin in PBS with 0.05% Tween-20. After blocking, the purified B3scdsFv-SEA was added to each well. PBS served as the negative control. The plate was incubated at 37 °C for 1 h. After being washed with PBST, the rabbit polyclonal antibody against SEA (1:1 000 diluted by PBST) was added and incubated at 37 °C for 1 h followed by incubation with HRP-coupled goat anti-rabbit IgG for 30 min. At last, the substrate TMB was applied for 3 min and the absorbance at 450 nm was measured when the reaction was stopped by the addition of 2 mol/L H2SO4.
Growth inhibition assay was performed in 96-well plates as described previously[11]. Briefly, 5×103 HT-29 cells were added to each well, followed by B3scdsFv-SEA dilutions and 4×104 effector cells (human peripheral blood T lymphocytes) in a total volume of 200 μL. Cells were cultured for 5 d at 37 °C in a humidified atmosphere containing 50 mL/L CO2. After the culture, the number of remaining tumor cells was determined using a non-radioactive cell proliferation assay kit (Promega, USA) and the A490 was measured using a SPECTRA max Plus spectrophotometer (Molecular Device Corp., USA). Data were expressed as percentage of tumor growth inhibition, which was calculated as (1-Atest/AC)×100%, where Atest indicates the absorbance of tumor cells grown in the presence of effector cells and B3scdsFv-SEA dilutions, AC indicates the absorbance of tumor cells grown in medium with the effector cells.
The stability of recombinant toxin was determined by incubating for different periods at 37 °C in PBS, and the remaining activity was determined by testing the binding to the HT-29 cell line.
The expression products in bacterial strain BL21 (DE3) pLysS was mainly found in the inclusion body as shown in Figure 2A. The relative mass of targeted protein was approximately 50 ku, and its expression level was approximately 30% of the total cellular protein as determined by TotalLab 2.01 software analysis. Western blotting was performed to test the identity of B3scdsFv-SEA. The rabbit polyclonal antibody could recognize the B3scdsFv-SEA protein at around 50 ku, but the same reaction was not observed at lane 2 (the negative control) (Figure 2B).
In order to generate protein with a native conformation, we solubilized and reduced the inclusion body in guanidine hydrochloride and DTT, and refolded the protein by dilution in a buffer containing arginine at 10 °C. The refolded product was purified through SP-sepharose column after concentration and buffer exchange.
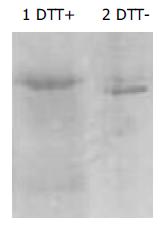
The fraction of B3scdsFv-SEA molecules could be determined by SDS-PAGE. Figure 3 shows, that the gel mobility of B3scdsFv-SEA was more rapid under non-reducing condition than under reducing condition, indicating that the disulfide bond might be formed during the refolding process.
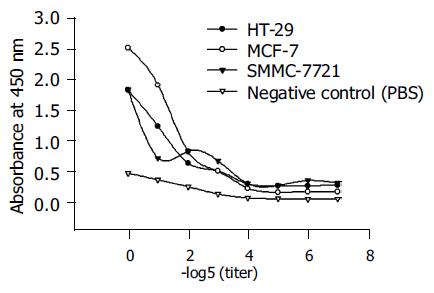
The binding affinity of B3scdsFv-SEA was measured on B3 antigen expressing cell lines HT-29, MCF-7, and SMMC-7721. Figure 4 shows, that the binding affinity increased with the protein concentration, and had a better binding ability on MCF-7 cells because the expression level of B3 antigen on MCF-7 was higher than that of the other two cell lines[12].
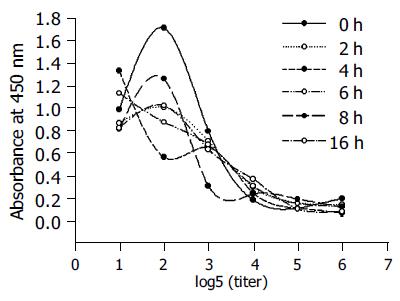
B3scdsFv-SEA was very stable at 37 °C in PBS. The binding activity of B3scdsFv-SEA at different time points was determined by ELISA. The recombinant toxin retained 85% of its activity after 16 h incubation at 37 °C (Figure 5).
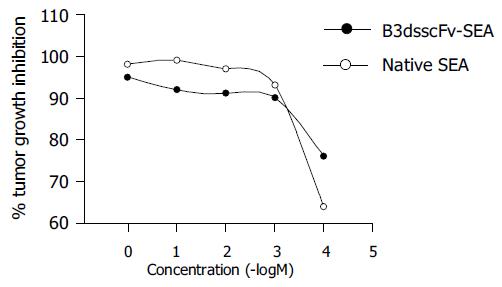
The toxicity of B3scdsFv-SEA to human colon carcinoma cell line HT-29 was determined by the MTT assay. The results of a representative experiment are shown in Figure 6. The maximum killing effect could achieve 95% when the protein concentration was 1 μg/mL. But the cytotoxic potency was a bit lower than native SEA. No killing effect on tumor cells was observed when the effector cells were added alone (data not shown).
The Fv fragment of an antibody can penetrate tumors more quickly than the intact antibody because of its small size[13]. Because of this advantage, different types of Fv fragments such as ScFv and dsFv have been extensively applied in tumor targeting therapy. But the instability of the ScFv and the low yield of dsFv become the obstacles in their clinical application. It was reported that the ScdsFv which has a peptide linker and a disulfide bond between VH and VL is stable at least after 1 wk exposure to human plasma at 37 °C, even under denaturing urea (6 mol/L) conditions, and it has a higher yield after renaturation than dsFv[8].
In this study, the ScdsFv of B3 mAb was fused to SEA (D227A). The fusion protein was expressed as inclusion body in E.coli. Small-scale preparations were performed in order to determine the optimal denaturing conditions, and we found that the maximum protein activity could be achieved in the presence of 0.5 mol/L L-Arg and 0.9 mmol/L GSSG in the refolding solution and the optimal denaturing temperature was 10 °C. The refolded products were purified to get rid of the renaturation agents, the purified B3scdsFv-SEA (D227A) had excellent binding affinity when incubated with B3 antigen positive tumor cell lines. The cytotoxic results indicate that the B3scdsFv-targeted superantigen suppresses the tumor cell growth successfully by activating the T lymphocytes. Moreover, the resulting protein is stable when incubated at 37 °C. All the SEA show that the binding and cytotoxic ability is not affected by the presence of both a linker and a disulfide bond between B3VH and B3VL.
In conclusion, B3scdsFv-targeted superantigen can be constructed in a bacterial system and this fusion protein shows its promise as a good reagent for cancer immunotherapy.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Kalland T, Dohlsten M, Abrahmsén L, Hedlund G, Björk P, Lando PA, Sundstedt A, Akerblom E, Lind P. Targeting of superantigens. Cell Biophys. 1993;22:147-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Hansson J, Ohlsson L, Persson R, Andersson G, Ilbäck NG, Litton MJ, Kalland T, Dohlsten M. Genetically engineered superantigens as tolerable antitumor agents. Proc Natl Acad Sci USA. 1997;94:2489-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Dohlsten M, Hedlund G, Akerblom E, Lando PA, Kalland T. Monoclonal antibody-targeted superantigens: a different class of anti-tumor agents. Proc Natl Acad Sci USA. 1991;88:9287-9291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Brinkmann U, Pai LH, FitzGerald DJ, Willingham M, Pastan I. B3(Fv)-PE38KDEL, a single-chain immunotoxin that causes complete regression of a human carcinoma in mice. Proc Natl Acad Sci USA. 1991;88:8616-8620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 149] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Pai LH, Batra JK, FitzGerald DJ, Willingham MC, Pastan I. Antitumor effects of B3-PE and B3-LysPE40 in a nude mouse model of human breast cancer and the evaluation of B3-PE toxicity in monkeys. Cancer Res. 1992;52:3189-3193. [PubMed] |
| 6. | Rajagopal V, Pastan I, Kreitman RJ. A form of anti-Tac(Fv) which is both single-chain and disulfide stabilized: comparison with its single-chain and disulfide-stabilized homologs. Protein Eng. 1997;10:1453-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Reiter Y, Brinkmann U, Jung SH, Lee B, Kasprzyk PG, King CR, Pastan I. Improved binding and antitumor activity of a recombinant anti-erbB2 immunotoxin by disulfide stabilization of the Fv fragment. J Biol Chem. 1994;269:18327-18331. [PubMed] |
| 8. | Bera TK, Onda M, Brinkmann U, Pastan I. A bivalent disulfide-stabilized Fv with improved antigen binding to erbB2. J Mol Biol. 1998;281:475-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Jung SH, Pastan I, Lee B. Design of interchain disulfide bonds in the framework region of the Fv fragment of the monoclonal antibody B3. Proteins. 1994;19:35-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Brinkmann U, Reiter Y, Jung SH, Lee B, Pastan I. A recombinant immunotoxin containing a disulfide-stabilized Fv fragment. Proc Natl Acad Sci USA. 1993;90:7538-7542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 134] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Dohlsten M, Lando PA, Björk P, Abrahmsén L, Ohlsson L, Lind P, Kalland T. Immunotherapy of human colon cancer by antibody-targeted superantigens. Cancer Immunol Immunother. 1995;41:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Pastan I, Lovelace ET, Gallo MG, Rutherford AV, Magnani JL, Willingham MC. Characterization of monoclonal antibodies B1 and B3 that react with mucinous adenocarcinomas. Cancer Res. 1991;51:3781-3787. [PubMed] |
| 13. | Kuan CT, Pastan I. Improved antitumor activity of a recombinant anti-Lewis(y) immunotoxin not requiring proteolytic activation. Proc Natl Acad Sci USA. 1996;93:974-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |