Published online Aug 21, 2005. doi: 10.3748/wjg.v11.i31.4895
Revised: January 23, 2005
Accepted: January 26, 2005
Published online: August 21, 2005
AIM: To investigate the combined effect of etoposide and radiation on CT26 colorectal adenocarcinoma implanted into BALB/c mice.
METHODS: We evaluated the radiosensitizing effect of etoposide on CT26 colorectal adenocarcinoma in a syngeneic animal model. BALB/c mice were subcutaneously implanted with CT26 cells and divided into four groups: control (intra-peritoneal saline2) group, etoposide (5 mg/kg intra-peritoneally2) group, radiation therapy (RT 5 Gy2 fractions) group, and combination therapy with etoposide (5 mg/kg intra-peritoneally 1 h before radiation) group.
RESULTS: Tumor growth was significantly inhibited by RT and combination therapy. The effect of combination therapy was better than that of RT. No significant changes were noted in body weight, plasma alanine aminotransferase, or creatinine in any group. The leukocyte count significantly but transiently decreased in the RT and combination therapy groups, but not in the etoposide and control groups. There was no skin change or hair loss in the RT and combination therapy groups.
CONCLUSION: Etoposide can sensitize CT26 colorectal adenocarcinoma in BALB/c mice to RT without significant toxicity.
- Citation: Liu CY, Liao HF, Wang TE, Lin SC, Shih SC, Chang WH, Yang YC, Lin CC, Chen YJ. Etoposide sensitizes CT26 colorectal adenocarcinoma to radiation therapy in BALB/c mice. World J Gastroenterol 2005; 11(31): 4895-4898
- URL: https://www.wjgnet.com/1007-9327/full/v11/i31/4895.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i31.4895
Colorectal cancer (CRC) is one of the most common malignancies worldwide, accounting for a significant percentage of cancer mortality. The incidence in both developing and developed countries has been increasing over the past few decades. Concurrent chemo-radiation therapy (CCRT) plays an important role in controlling CRC and palliating symptoms. This is particularly true when aiming for anal preservation in locally advanced disease, and it therefore offers an attractive alternative to surgery, the current mainstay of treatment[1].
Radiation therapy (RT), either as a definitive treatment along with chemotherapy for unresectable disease or as a post-operative adjuvant for resectable disease, plays an important role in the management of CRC[2,3]. However, large portions of the colorectum, small intestine, and urinary bladder are inevitably included within the radiation field in order to treat the areas of pelvic lymphatic drainage. Therefore, radiation-induced toxicity limits the use of RT in treating this malignancy, either alone or as part of a CCRT protocol. Moreover, some tumor clones are resistant to RT or chemotherapy. Currently, CCRT using a 5-fluorouracil (5-FU)-based regimen is the mainstay of CRC therapy. 5-FU is known to be a radiosensitizer, but some cancer cell clones are resistant to it[4,5]. Given these limitations, it is desirable to look for radiosensitizers that augment the efficacy of RT, thus allowing a lower RT dose, and have acceptably low toxicity.
Etoposide (VP-16) is a semi-synthetic derivative of the naturally occurring antibiotic podophyllotoxin, which poisons type II topoisomerase without binding to DNA[6]. Etoposide has been widely used in the treatment of lung and ovarian cancer, given either intravenously or orally[7-9]. The combination of etoposide and cisplatin given concurrently with RT for lung cancer has yielded promising results[10]. Etoposide-based CCRT also appears to be effective in newly diagnosed malignant glioma[11]. Chemotherapy with etoposide as a single agent, however, is not very successful in a phase II trial for colorectal cancer[12], which may explain why there is little interest in this drug for this disease. However, Shigematsu et al[13], reported, that a combination of low dose etoposide and radiation, arrests V79 (Chinese hamster fibroblasts) and T24 (human bladder cancer) cells in the G2/M phase of the cell cycle, decreasing their survival. Since G2/M is a radiosensitive phase of cell cycle, it is possible that etoposide functions as a radiosensitizer rather than a cytotoxic agent.
In this study, we investigated the combined effect of etoposide and radiation on CT26 colorectal adenocarcinoma implanted into BALB/c mice. Both the therapeutic effect and safety profile were evaluated.
CT26 cells, N-nitroso-N-methyl urethane-induced mouse colon carcinoma cells of BALB/c origin[14], were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in RPMI1640 medium (GIBCO, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal calf serum (Hyclone, Logan, UT, USA) at 37 °C in a humidified 50 mL/L CO2 incubator. The cell cultures were passaged every 2-3 d with TEG solution (0.25% trypsin, 0.1% EDTA and 0.05% glucose in Hanks balanced salt solution) and maintained in exponential growth. Male BALB/c mice, aged from 6 to 8 wk, were obtained from the National Laboratory Animal Center (Taipei, Taiwan) and housed in a rodent facility at 221 °C in a 12-h light-dark cycle. Four groups of animals of 9-11 mice each were implanted with CT26 cells (2105 cells) by subcutaneous injection into the right gluteal region. Treatment was started when the tumors grew to 0.5 cm in diameter. All experiments were performed in accordance with the regulations of the NIH Guide for the Care and Use of Laboratory Animals (DHHS publication no. NIH 85-23, revised 1996).
Etoposide was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). It was dissolved in dimethyl sulfoxide (DMSO) at 10 mmol/L as a stock solution and stored at -20 °C. For experimental use, the stock solution was diluted to the appropriate concentration with growth medium, with a final DMSO concentration of less than 0.1%. This concentration of DMSO has been previously proven to be nontoxic to cells.
There were four groups of mice in our study. The first group received intra-peritoneal (i.p.) saline for 2 consecutive days, and served as controls. The second group received daily i.p. injections of etoposide, 5 mg/kg for 2 consecutive days. The third group received RT, 5 Gy daily for 2 consecutive days. The fourth group received a combination of etoposide, followed by RT 1 h later. The doses of etoposide and RT were the same as used in the previous two groups. The mice were anesthetized with pentobarbital (50 mg/kg i.p.) before RT. The gluteal region, including both femurs and tumor, was irradiated with a total of 10 Gy in two daily fractions (6 MV photon beam at source-to-axis distance of 100 cm, dose rate 2.4 Gy/min) by an accelerator (Clinac 1800, Varian Associates Inc., CA, USA, dose rate 2.4 Gy/min). Two tissue-equivalent polystyrene plates (1.3 cm thick upward and 5.0 cm thick downward) were used to provide adequate build-up. Dosimetry was measured using a N30001 ionization chamber (PTW-FREIBURG, Germany) prior to radiation.
The total body weight of each mouse and size of implanted tumors were determined every other day by a single observer. Calipers were used to measure the largest (a) and smallest (b) diameter, and the tumor volume was estimated according to the formula 0.5ab2[19]. The leukocyte count was estimated by retro-orbital blood sampling every other day after treatment. Animals were killed when the first mouse expired after treatment. The plasma levels of alanine aminotransferase (ALT) and creatinine were measured with a SYNCHRON LX20 spectrophotometer (Beckman Coulter, San Diego, CA, USA) by heart blood sampling after being killed. Skin changes and hail loss in the mice were evaluated by a single observer after RT every other day.
Data were expressed as mean±SE or percentage. Analysis of variance was used to compare tumor size, body weight, ALT, and creatinine, and leukocyte count among the groups and controls. We used Sigma Stat software (version 2.03, SPSS Inc., Chicago, IL, USA) to perform the statistical analysis. P<0.05 was considered statistically significant.
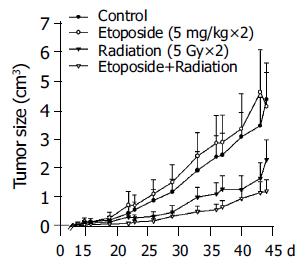
As shown in Figure 1, low-dose etoposide alone had no significant effect on tumor growth compared to the control group. However, RT alone and the combination therapy resulted in significant decrease in tumor size compared to controls (P<0.05). Combination therapy yielded the best results among the four groups. The tumor size at the end of the study in the combination therapy group was only 54% that of the RT group (1.27 cm3 vs 2.35 cm3).
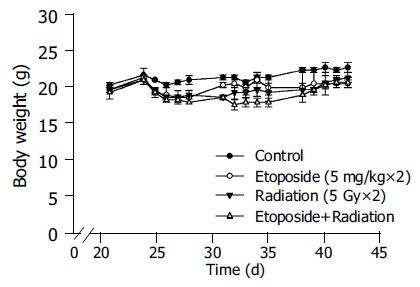
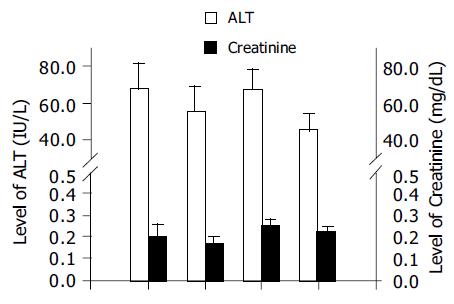
There were no significant changes in body weight in any of the four groups (Figure 2). Similarly, there were no significant differences in the plasma ALT and creatinine levels among the four groups (Figure 3). There was no obvious skin change or hair loss in any of the mice after RT or combination therapy except for tumor-related destruction.
The leukocyte count decreased significantly but transiently after treatment with RT or combination therapy (P<0.05). As shown in Figure 4, the nadir of the leukocyte count occurred 8 d after treatment. In contrast, the leukocyte counts in the control and etoposide groups had no evident change.
Our findings indicate that etoposide is a potent radiosensitizer in murine CT-26 colorectal adenocarcinoma cells without evident toxicity in vivo. This supports the contention that the role of etoposide in CRC treatment is as a radiosensitizer rather than as a cytotoxic agent.
Body weight and plasma ALT and creatinine were unaffected by any of the treatment modalities. The fact that transient leukopenia was found in both the RT and combination therapy groups but not in the etoposide group suggests that the bone marrow suppression may be caused by the radiation alone. The RT regimen used in this study may therefore needs to be adjusted to avoid myelosuppression. O’Dwyer et al[16], reported, that etoposide-related leukopenia is dose-dependent. The low dose we used was apparently successful in avoiding bone marrow suppression. Low-dose etoposide therefore may have little toxicity per se, but in our study, even this low dose appeared to have significant radiosensitizing activity.
One advantage of etoposide is that it comes in an oral form. Long term oral administration of etoposide improves the prognosis of ovarian cancer in patients who tolerate it well[8,17]. This suggests that oral etoposide might be a good candidate for a daily radiosensitizer. One study has shown that there is no significant difference between intravenous and oral administration of etoposide in terms of median response duration, time to progression, and survival when used to treat small cell lung cancer[18]. Shigematsu et al[13], also reported that the killing effect of low dose etoposide in vitro, seems to depend on the duration of exposure rather than the concentration. This duration-dependent rather than concentration-dependent also supports the use of lower dose etoposide as a radiosensitizer, as we found in our study.
Newer chemotherapeutic and biological agents, such as irinotecan (CPT-11)[19], oxaliplatin[20], and cyclooxygenase-2 inhibitors (e.g. celecoxib)[21] have been studied for their radiosensitizing potential in rectal cancer. These agents are particularly attractive because they have a favorable safety profile[22]. According to our results, low dose etoposide also appears to be safe and effective in the mouse model. It would therefore be worth conducting a clinical trial of etoposide-based CCRT for 5-FU resistant rectal cancer.
Although CCRT for CRC has promising clinical results, the mechanism of conventional radiosensitizers such as 5-FU remains unclear[23]. This is true for etoposide as well. Shigematsu et al[13], have reported a significant increase in cells in the G2/M phase after exposure to low-dose etoposide in vitro. This phenomenon is similar to our preliminary results (data not shown). Since cells in the G2/M phase are radiosensitive, etoposide may act to arrest CT26 cells in that phase, thus sensitizing them to RT. Another possibility is that the drug upregulates p53 and Bcl-2 expression, as has been reported in CT26 cells in vivo[24], an effect that may induce apoptosis. Apoptosis may play an important role in the mechanism of radiosensitization. In the future, we are planning to assess apoptosis in tumors by annexin V/propidium iodide or the TUNEL test.
In conclusion, the results of this study indicate that low-dose etoposide can sensitize CT26 cells to RT in vivo without evident toxicity. Further studies to identify the mechanism of etoposide-related radiosensitization are needed.
The authors wish to thank Dr. Mary Jeanne Buttrey for critical reading and correction of the manuscript.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Midgley R, Kerr D. Colorectal cancer. Lancet. 1999;353:391-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 277] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 2. | Fisher B, Wolmark N, Rockette H, Redmond C, Deutsch M, Wickerham DL, Fisher ER, Caplan R, Jones J, Lerner H. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst. 1988;80:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 796] [Cited by in RCA: 723] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 3. | O'Connell MJ, Martenson JA, Wieand HS, Krook JE, Macdonald JS, Haller DG, Mayer RJ, Gunderson LL, Rich TA. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331:502-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 761] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 4. | Garufi C, Brienza S, Pugliese P, Aschelter AM, Bensmaine F, Nisticò C, Giunta S, Caterino M, Giannarelli D, Cosimelli M. Overcoming resistance to chronomodulated 5-fluorouracil and folinic acid by the addition of chronomodulated oxaliplatin in advanced colorectal cancer patients. Anticancer Drugs. 2000;11:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Hughes LL, Luengas J, Rich TA, Murray D. Radiosensitization of cultured human colon adenocarcinoma cells by 5-fluorouracil: effects on cell survival, DNA repair, and cell recovery. Int J Radiat Oncol Biol Phys. 1992;23:983-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Ross W, Rowe T, Glisson B, Yalowich J, Liu L. Role of topoisomerase II in mediating epipodophyllotoxin-induced DNA cleavage. Cancer Res. 1984;44:5857-5860. [PubMed] |
| 7. | Johnson DH, Greco FA, Strupp J, Hande KR, Hainsworth JD. Prolonged administration of oral etoposide in patients with relapsed or refractory small-cell lung cancer: a phase II trial. J Clin Oncol. 1990;8:1613-1617. [PubMed] |
| 8. | Takeda S, Takada S, Kojima T, Kinoshita K, Sakamoto S. Oral etoposide therapy in stage III-IV ovarian carcinoma. Nihon Gan Chiryo Gakkai Shi. 1990;25:2562-2566. [PubMed] |
| 9. | Tucker RD, Ferguson A, Van Wyk C, Sealy R, Hewitson R, Levin W. Chemotherapy of small cell carcinoma of the lung with V.P. 16-213. Cancer. 1978;41:1710-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Lee JS, Komaki R, Fossella FV, Glisson BS, Hong WK, Cox JD. A pilot trial of hyperfractionated thoracic radiation therapy with concurrent cisplatin and oral etoposide for locally advanced inoperable non-small-cell lung cancer: a 5-year follow-up report. Int J Radiat Oncol Biol Phys. 1998;42:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Beauchesne P, Soler C, Boniol M, Schmitt T. Response to a phase II study of concomitant-to-sequential use of etoposide and radiation therapy in newly diagnosed malignant gliomas. Am J Clin Oncol. 2003;26:e22-e27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Perry MC, Moertel CG, Schutt AJ, Reitemeier RJ, Hahn RG. Phase II studies of dianhydrogalactitol and VP-16-213 in colorectal cancer. Cancer Treat Rep. 1976;60:1247-1250. [PubMed] |
| 13. | Shigematsu N, Kawata T, Ihara N, Kawaguchi O, Kutsuki S, Ishibashi R, Kubo A, Ito H. Effect of combined treatment with radiation and low dose etoposide on cell survival. Anticancer Res. 2001;21:325-328. [PubMed] |
| 14. | Brattain MG, Strobel-Stevens J, Fine D, Webb M, Sarrif AM. Establishment of mouse colonic carcinoma cell lines with different metastatic properties. Cancer Res. 1980;40:2142-2146. [PubMed] |
| 15. | Yang EB, Tang WY, Zhang K, Cheng LY, Mack PO. Norcantharidin inhibits growth of human HepG2 cell-transplanted tumor in nude mice and prolongs host survival. Cancer Lett. 1997;117:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | O'Dwyer PJ, LaCreta FP, Daugherty JP, Hogan M, Rosenblum NG, O'Dwyer JL, Comis RL. Phase I pharmacokinetic study of intraperitoneal etoposide. Cancer Res. 1991;51:2041-2046. [PubMed] |
| 17. | Yasumizu T, Kato J. Clinical trial of daily low-dose oral etoposide for patients with residual or recurrent cancer of the ovary or uterus. J Obstet Gynaecol (Tokyo 1995). 1995;21:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Johnson DH, Ruckdeschel JC, Keller JH, Lyman GH, Kallas GJ, Macdonald J, DeConti RC, Lee J, Ringenberg QS, Patterson WP. A randomized trial to compare intravenous and oral etoposide in combination with cisplatin for the treatment of small cell lung cancer. Cancer. 1991;67:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Wang DS, Ueno Y, Oyamada H, Kojima S. Enhancement of the antitumor effect of gamma-ray irradiation in combination with camptothecin against human colorectal adenocarcinoma. Biol Pharm Bull. 1996;19:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Gérard JP, Chapet O, Nemoz C, Romestaing P, Mornex F, Coquard R, Barbet N, Atlan D, Adeleine P, Freyer G. Preoperative concurrent chemoradiotherapy in locally advanced rectal cancer with high-dose radiation and oxaliplatin-containing regimen: the Lyon R0-04 phase II trial. J Clin Oncol. 2003;21:1119-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 150] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Kishi K, Petersen S, Petersen C, Hunter N, Mason K, Masferrer JL, Tofilon PJ, Milas L. Preferential enhancement of tumor radioresponse by a cyclooxygenase-2 inhibitor. Cancer Res. 2000;60:1326-1331. [PubMed] |
| 22. | Zhu AX, Willett CG. Chemotherapeutic and biologic agents as radiosensitizers in rectal cancer. Semin Radiat Oncol. 2003;13:454-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Lawrence TS, Blackstock AW, McGinn C. The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Semin Radiat Oncol. 2003;13:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 199] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 24. | Wang C, Yang YG, Wang XL. Expressions of hTERT, p53, c-erbB-2 and Bcl-2 in colonic adenocarcinoma mouse models with liver metastases: effects of classical traditional Chinese medicine prescriptions for promoting circulation and removing blood stasis. DiYi JunYi DaXue XueBao. 2004;24:758-760. [PubMed] |