Published online Aug 21, 2005. doi: 10.3748/wjg.v11.i31.4782
Revised: January 1, 2005
Accepted: January 5, 2005
Published online: August 21, 2005
AIM: Human β-defensin (HBD)-1 and HBD-2 are endogenous antimicrobial peptides. Unlike HBD-1, the HBD-2 expression is augmented by Helicobacter pylori (H pylori). We sought to determine HBD-1 and HBD-2 concentrations in gastric juice during H pylori infection.
METHODS: HBD-1 and HBD-2 concentrations were measured by radioimmunoassay in plasma and gastric juice of 49 H pylori-infected and 33 uninfected subjects and before and after anti-H pylori treatment in 13 patients with H pylori-associated gastritis. Interleukin (IL)-1β and IL-8 concentrations in gastric juice were measured by enzyme-linked immunosorbent assay (ELISA). Histological grades of gastritis were determined using two biopsy specimens taken from the antrum and corpus. Reverse phase high performance liquid chromatography (RP-HPLC) was used to identify HBD-2.
RESULTS: HBD-2 concentrations in gastric juice, but not in plasma, were significantly higher in H pylori-positive than -negative subjects, albeit the post-treatment levels were unchanged. Immunoreactivity for HBD-2 was exclusively identified in H pylori-infected mucosa by RP-HPLC. HBD-2 concentrations in gastric juice correlated with histological degree of neutrophil and mononuclear cell infiltration in the corpus. IL-1β levels correlated with those of IL-8, but not HBD-2. Plasma and gastric juice HBD-1 concentrations were similar in H pylori-infected and uninfected subjects.
CONCLUSION: Our results place the β-defensins, especially HBD-2, in the front line of innate immune defence. Moreover, HBD-2 may be involved in the pathogenesis of H pylori-associated gastritis, possibly through its function as immune and inflammatory mediator.
- Citation: Isomoto H, Mukae H, Ishimoto H, Nishi Y, Wen CY, Wada A, Ohnita K, Hirayama T, Nakazato M, Kohno S. High concentrations of human β-defensin 2 in gastric juice of patients with Helicobacter pylori infection. World J Gastroenterol 2005; 11(31): 4782-4787
- URL: https://www.wjgnet.com/1007-9327/full/v11/i31/4782.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i31.4782
Helicobacter pylori (H pylori) infection is the major cause of chronic gastritis and peptic ulcer disease and is a risk factor for gastric cancer[1,2]. The non-invasive organism colonizes the gastric epithelium and elicits specific antibodies against various immunogenic proteins derived from the bacteria[3]. H pylori-associated gastritis is characterized by intense infiltration of neutrophils and mononuclear cells into the lamina propria[4,5]. However, despite these humoral and cellular immune responses, the infection usually lasts a lifetime in the absence of antibiotic treatment[5]. Moreover, current anti-H pylori combination regimens are rather complicated and do not always result in cure of the infection because H pylori strains often develop resistance to antibiotic drugs[3,6].
Recently, various endogenous anti-microbial peptides have been identified as key elements of innate host defence against infection[7,9]. Defensins, single chain cationic peptides of molecular weight ranging from 3 000 to 4 500 Da, are one of the most extensively studied classes of such naturally occurring antibiotics[7,9]. They exhibit a wide variety of microbicidal activities against Gram-positive and -negative bacteria, mycobacteria, fungi and certain enveloped viruses[7,9]. Human defensins are divided into α- and β-defensins, based on the arrangements of three intra-molecular disulfide bridges[7,9]. At present, four members of β-defensins have been isolated in humans[7-11]. They are essentially synthesized in the epithelial compartment at various mucosal sites[7-11].
Recent in vitro studies showed the constitutive expression of human β-defensin (HBD)-1 and induced expression of HBD-2 in several gastric cancer cell lines in response to H pylori infection[12-14]. Exclusive enhancement of HBD-2 expression upon H pylori infection was also noted in patients with chronic gastritis[15,16]. However, there is no information on the secretion of HBD-2 into the gastric lumen in vivo or its concentrations in gastric juice during H pylori infection.
We have developed a sensitive, specific radioimmunoassay (RIA) for HBD[17]. Employing this assay system, we measured HBD-2 concentrations in gastric juice of H pylori-infected and uninfected individuals. This study paves the way for further understanding of the mechanisms involved in host immune response to this pathogen.
A total of 82 patients referred for diagnostic upper gastro-intestinal endoscopy between September 2002 and August 2003 were enrolled in the present study. The following exclusion criteria were applied for enrollment in the study. (1) The use of non-steroidal anti-inflammatory drugs, proton pump inhibitors, histamine H2-receptor antagonists or antibiotics within 4 wk prior to the present study; (2) History of severe concomitant diseases, upper gastro-intestinal surgery, peptic ulcer diseases and gastric cancer. On the day of endoscopy, blood samples were taken, transferred into tubes containing EDTA-2Na and aprotinin, centrifuged, plasma separated, and stored at -80 °C until assay.
At the beginning of endoscopy (XQ 200; Olympus Optical Co., Tokyo, Japan), a sample of the gastric juice was aspirated into collection tube containing EDTA-2Na and aprotinin using an aspiration instrument (PW-6P-1, Olympus) under endoscopic guidance. Gastric juice samples were immediately neutralized to pH 7.0 with 1 N NaOH and frozen at -80 °C until measurement. Two biopsy specimens were endoscopically obtained from both the antrum within 2 cm of the pyloric ring and the middle portion of the corpus along the greater curvature, fixed in 10% buffered formalin and embedded in paraffin. One was used for rapid urease test (Helicocheck, Otsuka Pharmaceutical Co., Tokushima, Japan) and another for histopathological and immunohistochemical assessments.
Fourteen patients with H pylori-associated gastritis were treated with eradication therapy consisting of lansoprazole (30 mg twice daily), amoxicillin (750 mg twice daily) and clarithromycin (400 mg twice daily) for 7 d[18]. Four weeks after cessation of the treatment, patients were examined by endoscopy again, and gastric juice and biopsy specimens were taken in a similar fashion to that performed before treatment.
Eradication of H pylori was considered successful when 13C-urea breath test was negative[18].
Paraffin-embedded biopsy specimens were cut into 4-µm thick sections and stained with hematoxylin and eosin. Each histological parameter of activity (neutrophils), chronic inflammation (mononuclear cells), glandular atrophy and intestinal metaplasia in the antrum and corpus of patients with H pylori infection was scored as 0, 1, 2 or 3 corresponding to none, mild, moderate or severe, respectively, based on the Sydney system[19,20]. Intestinal metaplasia was defined by the presence of goblet cells in glandular mucosa with Alcian blue (pH 2.50)/periodic acid-Schiff staining[21].
Furthermore, the density of H pylori colonization assessed with Giemsa staining was also scored from 0 to 3, based on the above classification system[19,20]. The biopsy specimens were examined blindly without knowledge of the results of β-defensins measurement.
H pylori status was assessed by serology (anti-H pylori immunoglobulin G antibody, HEL-p TEST, AMRAD Co., Melbourne, Australia), rapid urease test and histology with Giemsa staining. Patients were considered positive for H pylori infection when at least two of these examinations yielded positive results[20,22]. On the other hand, patients were defined as H pylori-negative, if all test results were negative[23].
The concentrations of interleukin (IL)-1β and -8 in gastric juice were measured as described previously[20,22,24]. The samples were assayed for total protein by a modified Lowry method, diluted to 0.5 mg/mL total protein concentration, and frozen at -80 °C until assay. Measurement of the two cytokines in the aliquots was performed using commercially available assay kits (Research and Diagnostics, Minneapolis, MN, USA), which employ the quantitative immunometric sandwich enzyme immunoassay technique. These assays were performed in duplicate according to the instructions provided by the manufacturer. In our study, inter- and intra-assay variabilities were <10%, respectively[20,22].
The concentrations of HBD-2 in plasma and gastric juice samples were measured by RIA established in our laboratory[17]. Briefly, full-length HBD-2 was synthesized using a peptide synthesizer (model 430, Applied Biosystems, Foster City, CA, USA) and purified by reverse phase high performance liquid chromatography (RP-HPLC). Synthetic HBD-2 was used for immunizing New Zealand white rabbits by multiple intracutaneous and subcutaneous injections. It was radio-iodinated and the 125I-labelled peptide was purified by RP-HPLC on a TSK ODS 120A column (Tosoh Co., Tokyo). The diluted sample or a standard peptide solution (100 μL) was incubated for 24 h with 100 μL antiserum diluent (final dilution 1:4 200 000). The 125I-labelled solution (16 000 cpm in 100 μL) was added and the mixture was incubated again for another 24 h. Normal rabbit serum and anti-rabbit IgG goat serum were then added and stored for 16 h. Bound and free ligands were separated by centrifugation. All procedures were performed at 4 °C and duplicate assays were carried out. Volumes of 0.5 mL plasma and 1-2 mL gastric juice were used to determine the levels of HBD-2. The concentrations of HBD-1 were also measured in similar fashions. The intra-assay and inter-assay coefficients of variation were <10%, respectively, in both the RIA analyses[15,23].
Chromatographic characterization of HBD-2 was performed as described previously[26,27]. Each 10 mg wet weight of non-cancerous mucosal tissue was sampled from surgically resected stomach of three patients with gastric cancer and H pylori infection. The samples were immediately homogenized and aliquots of homogenate supernatants, obtained by centrifugation (10 000 g for 10 min), were used for RP-HPLC on a TSK ODS SIL 120A column (Tosoh Co.). A linear gradient of acetonitrile (CH3CN) from 10% to 60% in 0.1% trifluoroacetic acid (pH 2.0) was used at a flow rate of 1.0 mL/min for 50 min. All fractions were analyzed for HBD-2 employing RIA.
Statistical analyses were performed using Fisher’s exact, χ2, Student’s t, Mann-Whitney U, Kruskal-Wallis, Spearman rank and Wilcoxon signed ranks tests, as appropriate. A P value of less than 0.05 was accepted as statistically significant. Data were expressed as mean±SD.
All examinations were conducted according to Good Clinical Practice and the Declaration of Helsinki, and were approved by the university ethics committees. All samples were obtained with written informed consent of the patients prior to their inclusion in the study. All experiments involving animals were approved by the ethics review committees for animal experimentation of participating universities.
The study population consisted of 49 H pylori-positive and 33 H pylori-negative subjects. They included 38 men and 44 women, with mean age of 50 years (range, 25-78 years). There were no significant differences between the H pylori-positive and -negative groups in background data on age, sex, body mass index, current tobacco use and alcohol intake (Table 1).
| H pylori-positive (n = 49) | H pylori-negative (n = 33) | |
| Mean age, ys (range) | 49.9 (25-72) | 49.6 (28-78) |
| Male/female | 24/25 | 14/19 |
| Smoker | 17 (34.7%) | 12 (36.4%) |
| Alcohol drinker | 16 (32.7%) | 10 (30.3%) |
| Body mass index (range) | 24.4 (17.1-29.0) | 23.7 (16.4-28.8) |
HBD-2 levels in gastric juice of patients with H pylori infection were significantly higher than those of H pylori-negative subjects (P<0.0005, Table 2), whereas infection had no significant impact on plasma concentrations of HBD-2 (Table 2). There were no significant differences in HBD-1 concentrations both in plasma and gastric juice with respect to H pylori status (Table 2). The levels of β-defensins in gastric juice did not correlate with those in plasma (correlation coefficient, r = 0.036 and -0.005 for each HBD-1 and HBD-2). In addition, there were no significant correlations between HBD-1 and HBD-2 levels in plasma (r = -0.077) and gastric juice (r = -0.092).
| H pylori-positive (n = 49) | H pylori-negative (n = 33) | P | |
| Plasma | |||
| HBD1 -1 levels (ng/mL) | 10.1±0.9 | 9.6±1.8 | NS |
| HBD-2 levels (pg/mL) | 276.2±61.0 | 257.2±56.0 | NS |
| Gastric juice | |||
| HBD-1 levels (pg/mL) | 384.2±101.5 | 357.9±79.6 | NS |
| HBD-2 levels (pg/mL) | 230.3±77.4 | 118.1±87.7 | <0.0005 |
| Interleukin 1β levels (mg/mL) | 53.6±16.9 | 5.3±4.4 | <0.05 |
| Interleukin 8 levels (pg/mL) | 17.9±3.1 | 8.6±1.7 | <0.005 |
There were significant differences in IL-1β and IL-8 concentrations in gastric juice between H pylori-positive and -negative subjects (P<0.05 and P<0.005, respectively, Table 2). HBD-2 concentrations in gastric juice did not correlate with IL-1β and IL-8 levels (r = -0.055 and r = 0.121, respectively). There was a positive correlation between IL-1β and IL-8 concentrations (r = 0.575, P<0.0001).
As shown in Table 3, HBD-2 concentrations in gastric juice correlated positively with the scores of activity and chronic inflammation in the corpus (P<0.005 and P<0.05, respectively), but not with glandular atrophy and intestinal metaplasia scores in the corpus. On the other hand, there were no significant correlations between HBD-2 concentrations and each score of gastritis in the antrum. In addition, H pylori density in the antrum and corpus did not correlate with HBD-2 concentrations in gastric juice.
| Correlation coefficient | P | |
| Antrum | ||
| Activity | 0.048 | NS |
| Chronic inflammation | 0.258 | NS |
| Glandular Atrophy | 0.148 | NS |
| Intestinal Metaplasia | -0.063 | NS |
| H pylori density | 0.011 | NS |
| Corpus | ||
| Activity | 0.499 | <0.005 |
| Chronic inflammation | 0.341 | <0.05 |
| Glandular Atrophy | 0.294 | NS |
| Intestinal Metaplasia | 0.081 | NS |
| H pylori density | 0.237 | NS |
Successful eradication of H pylori was achieved in 10 of 14 (71%) with the triple therapy. At 4 wk after completion of the 7-d treatment, HBD-2 concentrations in gastric juice of these patients were not significantly different from those measured before eradication therapy (Table 4). However, H pylori eradication was associated with a significant fall in IL-1β, and IL-8 levels of gastric juice compared with pre-treatment levels (P<0.05 and P<0.005, respectively, Table 4).
| Successful eradication of H pylori (n = 10) | Failed eradication (n = 4) | |||||
| Before therapy | After therapy | P | Before therapy | After therapy | P | |
| Gastric juice | ||||||
| HBD1-2 levels (pg/mL) | 182.2±57.6 | 210.1±66.4 | NS | 137.4±65.2 | 112.9±79.3 | NS |
| Interleukin 1β levels (pg/mL) | 17.7±10.2 | 0.5±0.3 | <0.05 | 88.5±80.9 | 64.7±64.1 | NS |
| Interleukin 8 levels (pg/mL) | 22.3±2.1 | 12.8±1.8 | <0.005 | 18.1±6.1 | 18.6±4.5 | NS |
In patients who were still infected with H pylori after antibiotic treatment, HBD-2, IL-1β and IL-8 levels in gastric juice remain unchanged compared to the pre-treatment concentrations (Table 4).
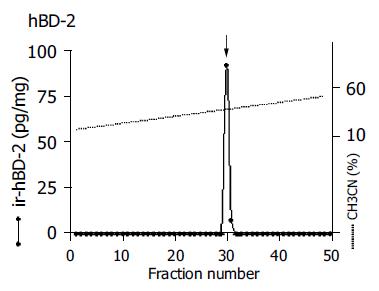
Immunoreactive HBD-2 was identified in the gastric mucosa infected with H pylori on RP-HPLC coupled with RIA (Figure 1). The peak was situated at a position identical to that of synthetic HBD-2 peptide. Each sample prepared yielded the same chromatographic pattern.
Recent studies have shown the inducible expression of HBD-2 messenger ribonucleic acid (mRNA) in response to H pylori infection in cultured gastric epithelial cells[12-14]. In clinical setting of gastritis, the HBD-2 mRNA and peptide expression is evidently increased in gastric mucosa infected with the organism[15,16]. There are a few in vitro observations of the secretion of HBD-2 peptide upon H pylori infection. George et al[26], showed its enhanced release in supernatants from infected gastric cancer cell lines by Western blotting. However, the actual concentrations of such antimicrobial peptides at the local site of human stomach during H pylori infection remain unknown. Our sensitive RIA system allowed us to determine the HBD-2 concentrations in gastric juice, as well as in other body fluids and blood[17,25]. Thus, we demonstrated, for the first time, that the HBD-2 levels in gastric juice of H pylori-infected patients were significantly higher than those of uninfected ones, suggesting its physio-pathological significance in the bacterial infection. In contrast, H pylori status did not have a significant impact on plasma HBD-2 levels. Immunoreactivity for HBD-2 was identified in H pylori-infected gastric mucosa by RT-HPLC combined with RIA. In agreement with the results of previous studies[15,16], immunohistochemical staining against anti-HBD-2 antibody within the infected mucosa was confined to the superficial epithelial cells. Taken together, we believe that H pylori infection stimulates the biosynthesis of HBD-2 in gastric mucosa, primarily in epithelia confronting this organism[15], and the product is mainly released into the gastric lumen.
In the present study, however, there was no significant difference in gastric juice, HBD-2 levels before and 4 wk after cure of the infection, and in certain cases, post-treatment HBD-2 levels were rather increased compared to the pre-treatment ones. In contrast, Hamanaka et al[15], reported that epithelial HBD-2 expression assessed by immunohistochemistry was markedly decreased in gastric specimens obtained 4 wk after eradication of H pylori, concomitant with the pronounced reduction of its transcripts to undetectable levels by reverse transcription-polymerase chain reaction (RT-PCR). These results must be interpreted within the context of studies limitations including their small sample sizes, differences in samples assessed-gastric biopsy tissues vs gastric juice- and diverse methodological approaches to HBD-2. Nevertheless, one might postulate the mingling of HBD-2 secreted by the other cells than gastric epithelial cells, such as esophageal squamous cells, in the gastric juice. In fact, more than 95% of biopsy specimens from the esophagus were positive for the β-defensin mRNA on RT-PCR analysis[16]. Several recent studies have shown that elimination of H pylori infection would lead to the development of gastro-esophageal reflux disease, probably in some cases, in association with post-treatment increase in acid secretion[27-29]. At present, however, there is no information available as to whether the esophageal HBD-2 expression is altered during this condition or upon such stimuli as refluxates with increased acidity.
We found the association of HBD-2 concentrations in gastric juice with histological degree of mucosal infiltration of neutrophils and mononuclear cells in the corpus, but not in the antrum. In line with this, Wehkamp et al[16], reported that H pylori colonization was significantly related to higher percentage of positive biopsies taken from the corpus with respect to HBD-2 transcripts. The sparse distribution and confined localization to cells of the apical foveolae of this peptide[15,16] may be attributable to the topographical differences in HBD-2 expression. In addition to the direct anti-microbial activity, evidence is accumulating for the role of defensins as immune and inflammatory mediators[7-9,30]. In fact, β-defensins display chemotactic activities for dendritic cells and T lymphocytes through the chemokine receptor CCR6[30]. It is possible that β-defensins, especially HBD-2, may be involved in the pathogenesis of H pylori-associated gastritis, in part, via their action as chemoattractant. Again, the HBD-2 levels in gastric juice can provide useful information on histological severity of corporal mucosal inflammation.
Recent in vitro studies have demonstrated that HBD-2 mRNA expression is stimulated by pro-inflammatory cytokines such as IL-1β and tumor necrosis factor α in various cell lines including gastric epithelial cells[9,12,13]. We have also reported a positive correlation between IL-1β and HBD-2 concentrations in broncho-alveolar lavage fluid of patients with pneumonia and diffuse panbronchiolitis[17,23]. In the present study, however, there was no significant correlation between gastric juice HBD-2 and IL-1β concentrations, whereas the IL-1β levels strongly correlated with those of IL-8. IL-1β is known to activate nuclear factor kappa B (NF-κB)[31], an important transcription factor regulating a plethora of genes including those of IL-8 and HBD-2[9,31,32]. In this regard, we demonstrated that mucosal IL-8 levels of patients with H pylori-associated gastritis correlated strongly with the grades of activated NF-κB expression in gastric epithelial cells[20,22]. Considered together, these data suggest that IL-8 expression is probably enhanced by IL-1β-induced NF-κB activation within the H pylori-infected mucosa. On the other hand, we suggest that the increased expression of HBD-2 in the gastric epithelium may result from the direct contact between this pathogen and epithelial cells in vivo.
H pylori status had no impact on HBD-1 concentrations in gastric juice, providing further evidence for its constitutive nature[12-14,16]. Since ingestion of contaminated food or water exposes the gastric mucosa to a wide variety of microbial pathogens, the constitutive expression of HBD-1 could play a ‘surveillance-like’ role during normal homeostasis of human stomach[33].
The in vitro minimal inhibitory concentrations against a panel of micro-organisms range from 0.1 to 100 μg/mL for defensin peptides[9]. Hamanaka et al[15], reported that the growth rate of cultured H pylori was suppressed by 50% on incubation with 0.3 μg/mL of HBD-2 and was completely inhibited at 30 μg/mL of this peptide. It remains to be determined whether β-defensins are microbicidal for the organism in gastric milieu in vivo. However, it is tempting to speculate that the H pylori strains that are able to establish persistent infection may be rather insensitive to such innate mucosal defense machinery.
In conclusion, we demonstrated the presence of significantly high levels of HBD-2 in gastric juice in patients with H pylori infection. The enhanced production/release of HBD-2 may contribute to the perpetuation of mucosal inflammation of the corpus. Our results highlight the importance of β-defensins, especially HBD-2, not only in innate defense but also in adaptive immune response to the organism.
Science Editor Guo SY Language Editor Elsevier HK
| 1. | Blaser MJ. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990;161:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 478] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 2. | Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu Rev Microbiol. 2000;54:615-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 398] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 3. | Zevering Y, Jacob L, Meyer TF. Naturally acquired human immune responses against Helicobacter pylori and implications for vaccine development. Gut. 1999;45:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Shimoyama T, Crabtree JE. Bacterial factors and immune pathogenesis in Helicobacter pylori infection. Gut. 1998;43 Suppl 1:S2-S5. [PubMed] |
| 5. | Allen LA. The role of the neutrophil and phagocytosis in infection caused by Helicobacter pylori. Curr Opin Infect Dis. 2001;14:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Isomoto H, Inoue K, Furusu H, Enjoji A, Fujimoto C, Yamakawa M, Hirakata Y, Omagari K, Mizuta Y, Murase K. High-dose rabeprazole-amoxicillin versus rabeprazole-amoxicillin-metronidazole as second-line treatment after failure of the Japanese standard regimen for Helicobacter pylori infection. Aliment Pharmacol Ther. 2003;18:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | van Wetering S, Sterk PJ, Rabe KF, Hiemstra PS. Defensins: key players or bystanders in infection, injury, and repair in the lung? J Allergy Clin Immunol. 1999;104:1131-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Chertov O, Yang D, Howard OM, Oppenheim JJ. Leukocyte granule proteins mobilize innate host defenses and adaptive immune responses. Immunol Rev. 2000;177:68-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Bals R. Epithelial antimicrobial peptides in host defense against infection. Respir Res. 2000;1:141-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 328] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 10. | Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001;276:5707-5713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1017] [Cited by in RCA: 1004] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 11. | García JR, Krause A, Schulz S, Rodríguez-Jiménez FJ, Klüver E, Adermann K, Forssmann U, Frimpong-Boateng A, Bals R, Forssmann WG. Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001;15:1819-1821. [PubMed] |
| 12. | O'Neil DA, Cole SP, Martin-Porter E, Housley MP, Liu L, Ganz T, Kagnoff MF. Regulation of human beta-defensins by gastric epithelial cells in response to infection with Helicobacter pylori or stimulation with interleukin-1. Infect Immun. 2000;68:5412-5415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 13. | Bajaj-Elliott M, Fedeli P, Smith GV, Domizio P, Maher L, Ali RS, Quinn AG, Farthing MJ. Modulation of host antimicrobial peptide (beta-defensins 1 and 2) expression during gastritis. Gut. 2002;51:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Uehara N, Yagihashi A, Kondoh K, Tsuji N, Fujita T, Hamada H, Watanabe N. Human beta-defensin-2 induction in Helicobacter pylori-infected gastric mucosal tissues: antimicrobial effect of overexpression. J Med Microbiol. 2003;52:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Hamanaka Y, Nakashima M, Wada A, Ito M, Kurazono H, Hojo H, Nakahara Y, Kohno S, Hirayama T, Sekine I. Expression of human beta-defensin 2 (hBD-2) in Helicobacter pylori induced gastritis: antibacterial effect of hBD-2 against Helicobacter pylori. Gut. 2001;49:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Wehkamp J, Schmidt K, Herrlinger KR, Baxmann S, Behling S, Wohlschläger C, Feller AC, Stange EF, Fellermann K. Defensin pattern in chronic gastritis: HBD-2 is differentially expressed with respect to Helicobacter pylori status. J Clin Pathol. 2003;56:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Hiratsuka T, Nakazato M, Date Y, Ashitani J, Minematsu T, Chino N, Matsukura S. Identification of human beta-defensin-2 in respiratory tract and plasma and its increase in bacterial pneumonia. Biochem Biophys Res Commun. 1998;249:943-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 104] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Isomoto H, Inoue K, Furusu H, Nishiyama H, Shikuwa S, Omagari K, Mizuta Y, Murase K, Murata I, Kohno S. Lafutidine, a novel histamine H2-receptor antagonist, versus lansoprazole in combination with amoxicillin and clarithromycin for eradication of Helicobacter pylori. Helicobacter. 2003;8:111-119. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Price AB. The Sydney System: histological division. J Gastroenterol Hepatol. 1991;6:209-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 655] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 20. | Isomoto H, Miyazaki M, Mizuta Y, Takeshima F, Murase K, Inoue K, Yamasaki K, Murata I, Koji T, Kohno S. Expression of nuclear factor-kappaB in Helicobacter pylori-infected gastric mucosa detected with southwestern histochemistry. Scand J Gastroenterol. 2000;35:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Morini S, Zullo A, Hassan C, Lorenzetti R, Stella F, Martini MT. Gastric cardia inflammation: role of Helicobacter pylori infection and symptoms of gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:2337-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Isomoto H, Mizuta Y, Miyazaki M, Takeshima F, Omagari K, Murase K, Nishiyama T, Inoue K, Murata I, Kohno S. Implication of NF-kappaB in Helicobacter pylori-associated gastritis. Am J Gastroenterol. 2000;95:2768-2776. [PubMed] |
| 23. | Isomoto H, Furusu H, Morikawa T, Mizuta Y, Nishiyama T, Omagari K, Murase K, Inoue K, Murata I, Kohno S. 5-day vs. 7-day triple therapy with rabeprazole, clarithromycin and amoxicillin for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2000;14:1619-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Isomoto H, Wang A, Mizuta Y, Akazawa Y, Ohba K, Omagari K, Miyazaki M, Murase K, Hayashi T, Inoue K. Elevated levels of chemokines in esophageal mucosa of patients with reflux esophagitis. Am J Gastroenterol. 2003;98:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Hiratsuka T, Mukae H, Iiboshi H, Ashitani J, Nabeshima K, Minematsu T, Chino N, Ihi T, Kohno S, Nakazato M. Increased concentrations of human beta-defensins in plasma and bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis. Thorax. 2003;58:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | George JT, Boughan PK, Karageorgiou H, Bajaj-Elliott M. Host anti-microbial response to Helicobacter pylori infection. Mol Immunol. 2003;40:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Labenz J, Blum AL, Bayerdörffer E, Meining A, Stolte M, Börsch G. Curing Helicobacter pylori infection in patients with duodenal ulcer may provoke reflux esophagitis. Gastroenterology. 1997;112:1442-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 410] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 28. | Fallone CA, Barkun AN, Friedman G, Mayrand S, Loo V, Beech R, Best L, Joseph L. Is Helicobacter pylori eradication associated with gastroesophageal reflux disease? Am J Gastroenterol. 2000;95:914-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Iijima K, Ohara S, Sekine H, Koike T, Kato K, Asaki S, Shimosegawa T, Toyota T. Changes in gastric acid secretion assayed by endoscopic gastrin test before and after Helicobacter pylori eradication. Gut. 2000;46:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Raj PA, Dentino AR. Current status of defensins and their role in innate and adaptive immunity. FEMS Microbiol Lett. 2002;206:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 157] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3573] [Cited by in RCA: 3590] [Article Influence: 128.2] [Reference Citation Analysis (0)] |
| 32. | Wada A, Ogushi K, Kimura T, Hojo H, Mori N, Suzuki S, Kumatori A, Se M, Nakahara Y, Nakamura M. Helicobacter pylori-mediated transcriptional regulation of the human beta-defensin 2 gene requires NF-kappaB. Cell Microbiol. 2001;3:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Zhao C, Wang I, Lehrer RI. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996;396:319-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 429] [Article Influence: 14.8] [Reference Citation Analysis (0)] |