Published online Aug 14, 2005. doi: 10.3748/wjg.v11.i30.4727
Revised: July 20, 2004
Accepted: July 22, 2004
Published online: August 14, 2005
AIM: To determine the prevalence of porcine endogenous retrovirus (PERV) in various pig breeds raised in China including Chinese experimental mini-pigs by PERV-reverse transcriptase (PERV-RT enzyme). Moreover, the potential for infection of PERV was investigated in patients treated with a bioreactor based on porcine liver cells (n = 3).
METHODS: Pig serum, liver and muscle cell-free supernatants were collected from various Chinese pig breeds. Porcine hepatocytes were isolated with a two-step perfusion method. Three patients with acute or chronic liver failure were treated with a bioartificial liver support system (BALSS) for 8-12 h and serum samples were collected from the patients before, immediately after and 30 d after treatment.
RESULTS: The activities of PERV-RT enzyme in pig liver and muscle cell-free supernatants were higher than in normal human controls. PERV-TR enzyme activity did not increase in patients before and after 1 mo of treatment. PERV-RT activities were not significantly different when compared with pre-treatment group (1.544 ± 0.155576), the post-treatment groups (1.501 ± 0.053507, 1.461 ± 0.033808 and 1.6006667 ± 0.01963 for 0, 14 and 30 d post-treatment, respectively, P > 0.05), and normal control group (1.440 ± 1.0641, P > 0.05). RT enzyme activity in Chinese experimental mini-pigs was higher than in normal human control group (1.440 ± 1.0641 U/mL, P < 0.05), and not significantly different (P > 0.05) when compared with the pig breeds except in the muscle supernatants. All the samples including muscle and liver cell supernatants from the Chinese mini-experimental pigs and the four domestic Chinese pig breeds contained PERVs.
CONCLUSION: These results suggest that the risk of PERV infection through BALSS containing porcine liver cells without immunosuppressants may be quite low. Although there were PERVs in Chinese experimental mini-pigs and porcine liver cell culture suspensions, we did not find any evidence of persistent PERV infection in patients treated with this porcine hepatocyte-based bioartificial liver.
- Citation: Liu Q, Liu Z, Dalakas E. Prevalence of porcine endogenous retrovirus in Chinese pig breeds and in patients treated with a porcine liver cell-based bioreactor. World J Gastroenterol 2005; 11(30): 4727-4730
- URL: https://www.wjgnet.com/1007-9327/full/v11/i30/4727.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i30.4727
Acute or chronic liver failure is still one of the most challenging clinical syndromes in modern medicine. Survival rate in patients with acute or chronic liver failure caused by hepatitis B virus (HBV) is less than 20%[1]. Various non-biological approaches including plasma exchange, hemodialysis and hemofiltration have had limited success, presumably because of the role of the synthetic and metabolic functions of the liver that are inadequately replaced in these systems. The liver is an organ with an extensive regenerative potential and has a remarkable capacity to meet replacement demands of cellular loss[2] If a system is designed to maintain normal liver functions for a bridging period until liver transplantation or liver recovery, then the survival rate of patients with acute liver failure (ALF) could be dramatically improved. Bioartificial liver support systems, which combine living cells of the liver in an extracorporeal circuit, have been successfully used in primary clinical trials and serve as a potent therapy and bridge to liver transplantation[3]. The shortage of human organs to be used for bioreactors and the lack of safe and effective human liver cell lines have resulted in pigs becoming an important hepatic cell source. Primary porcine hepatocytes are most commonly used in devices undergoing pre-clinical and clinical evaluation. However, using these cells may be associated with the risk of transmission of PERVs[4]. PERVs are present in the genome of all pigs and are able to infect human cells in vitro[5]. However, the prevalence of PERV in Chinese pig breeds and whether PERVs infect patients who undergo bioartificial liver treatments based on Chinese experimental mini-pig liver cells remains unclear. The objective of the study was to detect the prevalence of PERVs in the serum, liver and muscle cell-free supernatants of different pig breeds and in the serum of patients with acute or chronic liver failure, treated with a bioreactor based on porcine liver cells
Pig liver and muscle cell-free supernatant collection Muscle and liver samples (20 g) were collected from three Chinese experimental mini-pigs (the Institute of Experimental Animals, Beijing Agriculture University, China), weighing 10-15 kg each and from four domestic Chinese pigs of different breeds (Taihu pig, Wuzhishan pig, Dabai pig and Dahei pig-Beijing pig market), weighing approximately 60 kg each. The samples were taken and ground, pulverized with an SPS-3 Ultrasonic Pulverizer (working frequency 23 kHz; pulse 50% for 5 min). The pulverized samples were collected and centrifuged for 3 min at 80 r/min at room temperature. The cell-free supernatant was collected and resuspended in a solution provided by the C-type RT activity kit (Cavidi Tech AB, Uppsala, Sweden). The solution was then incubated for 30 min at room temperature and stored at -70°C until used.
Serum samples collection Serum samples (1.5 mL) were collected from domestic Chinese pigs (n = 4) and Chinese experimental mini-pigs (n = 3). Human serum samples (1.5 mL) were collected from normal adult controls (n = 4) and from patients (n = 3) undergoing bioartificial liver treatments with samples taken at pre-treatment, d 0, 14 and 30 post-treatment. All serum samples were stored at -70°C until used.
Pig hepatocytes were harvested from three Chinese experimental mini-pigs using a two-step in situ collagenase perfusion technique that was modified from the original method developed by Aiken[6]. The pig was initially anesthetized with ketamine (40 mg/kg). The liver was first perfused in vivo with oxygenated perfusion solution I (143 mmol/L sodium chloride, 6.7 mmol/L potassium chloride, 10 mmol/L Hepes, and 1 g/L EDTA, pH 7.4) at 300 mL/min for 20-40 min. The liver was then perfused ex vivo at 300 mL/min with oxygenated perfusion solution II (100 mmol/L Hepes, 67 mmol/L sodium chloride, 6.7 mmol/L potassium chloride, 4.8 mmol/L calcium chloride, 10 mL/L bovine albumin, and 1 g/L collagenase-D (Sigma), pH 7.6). Once the liver was visually dissolved (after 20-30 min), it was broken and irrigated with cold William’s E medium (Gibco) supplemented with 15 mmol/L Hepes, 0.2 U/mL insulin, 2 mmol/L L-glutamine (Gibco), 100 U/mL penicillin, and 100 mg/mL streptomycin. The released cells were filtered through nylon mesh with 100-μm openings and washed via three centrifugations (50 r/min) and resuspended in William’s E medium. Viability for the harvests as determined using trypan blue exclusion ranged from 90%.to 95%.
Three patients (2 males, 1 female, age 32-54) suffering from acute on chronic liver failure were recruited into the study. Informed consent was obtained from all the patients or their relatives and approved by the Beijing Youan Hospital Ethics Committee. All the patients involved in the study were treated with HBL (Cell Biotech Bioartificial Liver BIOLIV A3 device) in our center from March 2000 to June 2001. The criteria[7] for acute or chronic liver failure are as follows: (1) Evidence of chronic liver disease (chronic hepatitis and cirrhosis; cutaneous features of chronic liver disease; investigations demonstrating splenomegaly and hypoalbuminemia; evidence of chronic hepatitis/cirrhosis; chronic HBV carrier status by liver biopsy; chronic HBV/ HCV overlapping with HAV, HDV, HEV or other viral infection). (2) All of the following: Prolonged prothrombin time and PTA < 40%, bilirubin rise > 17.1 μmol/L per day or total bilirubin >171 μmol/L, Grade III/IV hepatic encephalopathy and ascites alone, or both simultaneously.
Liver and muscle cell-free supernatants and serum samples filtered through membranes (0.45-µm pore size) were analyzed for RT activity using the C-type RT activity assay (Cavidi Tech AB, Uppsala, Sweden) according to the instructions of the manufacturer (protocol B). One hundred and sixty microliters of sample dilution buffer was added to all wells of 96-well microtiter plate. Then 40 µL of various samples was added to wells. A series of MuLV RT standard dilutions served as the positive controls, with normal human serum and sample dilutions as the negative controls. The PolyA Plate was sealed with adhesive tape. The plate was incubated at 33°C for 3 h for the first plate and overnight for the second plate on an orbital shaker set at gentle agitation. The PolyA Plate was washed according to the protocol. Residual fluid in the wells was removed by tapping the plate upside down on absorbing cloth or paper. Two hundred microliters of AP substrate solution was added to each well of the PolyA plate and incubated at room temperature under dark cover on an orbital shaker set at gentle agitation. The filter of the plate reader was set at 405 nm. The absorbance of every well in the plate was read.
Data were expressed as the mean±SD. An unpaired Student’s t test was used to compare mean between two groups and ANOVA was used for multiple comparisons. P < 0.05 was considered significant.
Freshly isolated porcine hepatocytes were assessed for viability and function by trypan blue dye exclusion. The yield of cells was 5 × 109 cells/liver and cell viability was (95 ± 5)%. During HBL treatment, porcine hepatocyte viability was monitored every 30 min and viability was maintained at 80%.
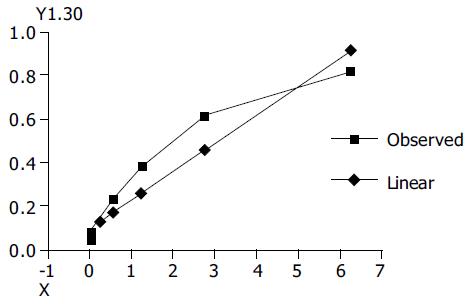
Standard curve and regression analysis The regression line was calculated with the use of SPSS10.0 software. A strong correlation was found between absorbance (A405) and the various concentrations of MuLV RT standard dilution (Figure 1). The regression equation was obtained as follows: Y = 0.098 617-0.131 297X, the relative coefficient (r) was 0.954 91, the regression coefficient comparison was done by an unpaired Student’s t test (t = 3.795, P < 0.0035) and both the absorbance and concentrations of MuLV RT were linear. RT activity for wells giving an A405 within the linear measuring range of the reader was determined by taking the A values of the samples into the regression equation. The results of each sample’s RT activity are shown in Tables 1 and 2.
| Pre-treatment | 0 d after treatment | 14 d after treatment | 30 d after treatment | |
| Patient 1 | 1.366 | 1.54 | 1.5 | 1.612 |
| Patient 2 | 1.612 | 1.44 | 1.44 | 1.612 |
| Patient 3 | 1.654 | 1.523 | 1.443 | 1.578 |
| Mean±SD | 1.544 ± 0.1555761 | 1.501 ± 0.0535071 | 1.461 ± 0.0338081 | 1.6006667 ± 0.019631 |
| Normal control (Mean±SD) | 1.441 ± 0.102 | 1.451667 ± 0.1198012 | 1.525667 ± 0.0850312 | 1.468333 ± 0.0850312 |
| Musclesupernatants | Liversupernatants | Serum | |
| Chinese experimental | 2.34 | 2.04 | 2.49 |
| mini-pig1 | |||
| Chinese experimental | 2.53 | 2.35 | 2.48 |
| mini-pig2 | |||
| Chinese experimental | 2.46 | 2.38 | 2.54 |
| mini-pig3 | |||
| mean±SD | 2.443333 ± 0.09609a | 2.256667 ± 0.188237a | 2.503333 ± 0.032146a |
| Taihu pig | 4 | 3.4 | 3.44 |
| Wuzhishan pig | 3.7 | 4.3 | 2.56 |
| Dabai pig | 3.49 | 3.08 | 2.95 |
| Dahei pig | 3.39 | 3.77 | 3.22 |
| mean±SD | 3.4950 ± 1.43875 | 3.6375 ± 0.523983 | 3.0425 ± 0.378979 |
| Human normal control | - | - | 1.440 ± 1.0641 |
Serum RT activities result from the three patients were: 1.544 ± 0.155576 pre-treatment, 1.501 ± 0.053507 at 0 d post-treatment, 1.461 ± 0.033808 at 14 d post-treatment and 1.6006667 ± 0.01963 at 30 d post-treatment. The difference of PERV-RT activities was not significant between the pre-treatment, post-treatment and normal control groups (Table 1). The patients treated for acute hepatic failure with a bioreactor based on porcine cells were shown to be free of PERV infections, with a follow-up period of 1 mo.
RT activities results from the Chinese experimental mini-pigs were 2.443333 ± 0.09609 U/mL in muscle cell-free supernatants, 2.256667 ± 0.188237 U/mL in liver cell-free supernatants and 2.503333 ± 0.032146 U/mL in serum, respectively. RT activities in the four domestic pig breeds were 3.645 ± 0.205 U/mL in muscle cell-free supernatants, 3.6375 ± 0.523983 U/mL in liver cell-free supernatants and 3.0425 ± 0.378979 U/mL in serum, respectively. Serum RT activity in the Chinese experimental mini-pigs was higher than in normal human control group 1.440 ± 1.0641 U/mL (P < 0.05), but not significantly different from the domestic pig breeds (P>0.05). RT activity in the muscle cell-free supernatant in the Chinese experimental mini-pigs was higher than that of the domestic pig breeds (P < 0.05) (Table 2). All of the samples including muscle and liver cell-free supernatants from either the Chinese mini-experimental pigs or the four domestic Chinese pigs demonstrated high PERV-RT activity, implicating that PERVs not only existed in these tissues but was also actively replicating.
According to the literature published from 1964 to 2000, a total of 270 patients worldwide with acute, sub-acute or chronic liver failure have been treated by 527 extracorporeal liver perfusions in 49 medical centers with livers from six different animal species, the pig being the most frequently used source[8]. In addition, swine organs are amongst the most likely source species of xenografts for clinical use to date[9-12]. The extracorporeal pig liver perfusion might provide the opportunity for a virus to evolve into a pathogen that can be transmitted from one individual to another efficiently enough to create a new epidemic disease. PERVs are a special risk, because they are part of the normal genetic material of mammalian cells except humans. Laboratory assays have been developed which target many aspects of the PERV replication/transmission cycle: PERV pro-viral DNA sequences, mRNA expression, RT activity, and the presence of proteins, as well as the detection of anti-PERV immune responses[13-15]. Among various assays, only enzymatic RT-activity is highly sensitive and specific for all PERV-A, PERV-B and PERV-C types, since RT is a generic marker of retroviruses. The presence of RT activity can be used to identify the presence of virus particles and, failure to detect it in these patient’s sera points to the absence of any other, unrecognized retrovirus of porcine origin[16].
This study showed that the RT activities from four domestic pig breeds were 2.445 U/mL in muscle cell-free supernatants, 2.508 U/mL in liver cell-free supernatants and 2.264 U/mL (range 2.048-2.387 U/mL) in serum respectively. All samples from the Chinese experimental mini-pigs and four domestic pig breeds contained PERVs that actively replicated in the tissues.
Breeding and keeping pigs under specific-pathogen-free or qualified-pathogen-free conditions is generally assumed to reduce the potential risk of transmitting exogenous viral, bacterial, fungal, and parasitic agents by xenotransplantation. Viral transmission, in a particularPERV is still a major obstacle, since PERVs are capable of infecting human cells in vitro. In a worse scenario, it is feared that PERV transmission to humans might be the starting point of a man-made pandemic threatening the health not only of BLASS patients, but also of the general public. A recent example of a rapid worldwide spread of an animal-borne disease was highlighted with the outbreak of severe acute respiratory syndrome (SARS), which spread in a matter of weeks from mainland China and Hong Kong to Vietnam, Singapore, Australia, Europe, and North America. This virus was the “common cold” virus of the Himalayan masked palm civet, an exotic species sold at live-animal markets in southern China where their meat is considered as a traditional delicacy. The transmission of SARS from animal to humans was traced back to civet chefs from Guangdong Province who contracted the disease, while handling infected meat.
The diameter of the 3A bioreactor membrane pore is about 200 nm and the diameter of PERV RNA is about 100 nm. Thus, it is quite possible for the PERV to pass through the bioreactor membrane. However, we did not find any evidence of infection with PERV in the patient’s serum soon after treatment or within 1 mo of follow-up. Like all investigations published to date, in patients who have been treated with porcine cells, tissues and organs, there has been no evidence for PERV infection. Although the results are encouraging, it has to be stated that the human immune system through a variety of specific and non-specific mechanisms can clear PERV particles effectively. As humans do not express functional α1, 3-galactosyl transferase, high titers of natural anti-gal-antibodies develop in their circulation because of continuous contact with gal-α1, 3-gal bearing microorganisms. These pre-formed or natural antibodies also effectively neutralize the α-gal-bearing γ-retroviruses including PERV. Since PERVs are produced by pig cells that have gal-α1, 3-gal-epitopes, they are sensitive to serum-mediated virolysis via the classical complement pathway in vivo. Thus, if immunosuppressed patients are treated with porcine liver cell bioreactor therapy then the potential risk of PERV infection will enhance. In this study the patient’s sera were not in direct contact with the pig cells and the time of treatment was limited to only 8-10 h. Clearly, with an increase in the duration of treatment by BLASS, the potential for PERV transmission will also increase. Therefore, it remains to be determined whether PERV transmission might occur in future trials and necessitates the continuation of strict monitoring regimens. The ability to monitor clinical trials closely using these assays will be of great benefit to the microbiological safety of clinical BLASS and xenotransplantation. This study sets one possible standard for laboratory surveillance of PERV infection after exposure to cellular xenografts from pigs.
Science Editor Zhu LH and Li WZ Language Editor Elsevier HK
| 1. | Schiødt FV, Davern TJ, Shakil AO, McGuire B, Samuel G, Lee WM. Viral hepatitis-related acute liver failure. Am J Gastroenterol. 2003;98:448-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Demetriou AA, Brown RS, Busuttil RW, Fair J, McGuire BM, Rosenthal P, Am Esch JS, Lerut J, Nyberg SL, Salizzoni M. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004;239:660-67; discussion 660-67;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 407] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 4. | Irgang M, Sauer IM, Karlas A, Zeilinger K, Gerlach JC, Kurth R, Neuhaus P, Denner J. Porcine endogenous retroviruses: no infection in patients treated with a bioreactor based on porcine liver cells. J Clin Virol. 2003;28:141-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Martin U, Kiessig V, Blusch JH, Haverich A, von der Helm K, Herden T, Steinhoff G. Expression of pig endogenous retrovirus by primary porcine endothelial cells and infection of human cells. Lancet. 1998;352:692-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 229] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Aiken J, Cima L, Schloo B, Mooney D, Johnson L, Langer R, Vacanti JP. Studies in rat liver perfusion for optimal harvest of hepatocytes. J Pediatr Surg. 1990;25:140-14; discussion 140-14;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Chinese Association for liver diseases and for contagious and parasitic disease. Guideline of prevention and treatment of virus hepatitis. Zhonghua Ganzangbing Zazhi. 2000;6:324. |
| 8. | Pascher A, Sauer IM, Hammer C, Gerlach JC, Neuhaus P. Extracorporeal liver perfusion as hepatic assist in acute liver failure: a review of world experience. Xenotransplantation. 2002;9:309-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | MacKenzie DA, Hullett DA, Sollinger HW. Xenogeneic transplantation of porcine islets: an overview. Transplantation. 2003;76:887-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Denner J. Porcine endogenous retroviruses (PERVs) and xenotransplantation: screening for transmission in several clinical trials and in experimental models using non-human primates. Ann Transplant. 2003;8:39-48. [PubMed] |
| 11. | Magre S, Takeuchi Y, Bartosch B. Xenotransplantation and pig endogenous retroviruses. Rev Med Virol. 2003;13:311-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Mollnes TE, Fiane AE. Role of complement in xenotransplantation. Allergy. 2002;57 Suppl 72:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Kuddus R, Patzer JF, Lopez R, Mazariegos GV, Meighen B, Kramer DJ, Rao AS. Clinical and laboratory evaluation of the safety of a bioartificial liver assist device for potential transmission of porcine endogenous retrovirus. Transplantation. 2002;73:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Switzer WM, Shanmugam V, Chapman L, Heneine W. Polymerase chain reaction assays for the diagnosis of infection with the porcine endogenous retrovirus and the detection of pig cells in human and nonhuman recipients of pig xenografts. Transplantation. 1999;68:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Clémenceau B, Jégou D, Martignat L, Saï P. Long-term follow-up failed to detect in vitro transmission of full-length porcine endogenous retroviruses from specific pathogen-free pig islets to human cells. Diabetologia. 2001;44:2044-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Blusch JH, Patience C, Martin U. Pig endogenous retroviruses and xenotransplantation. Xenotransplantation. 2002;9:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |