Published online Aug 14, 2005. doi: 10.3748/wjg.v11.i30.4697
Revised: December 20, 2004
Accepted: December 23, 2004
Published online: August 14, 2005
AIM: To assess the extent of micronutrient and oxidative stress in blood and to examine their linkages with viral loads in chronic hepatitis C patients.
METHODS: Hepatitis C virus (HCV)-RNA levels were quantified in the serum from 37 previously untreated patients with chronic hepatitis C. The plasma and erythrocyte micronutrients (zinc, selenium, copper, and iron) were estimated, and malondialdehyde (MDA) contents were determined as a marker to detect oxidative stress. Antioxidant enzymes, superoxide dismutase (SOD), glutathione peroxidase (GPX) and glutathione reductase (GR) activities in blood were also measured. The control group contained 31 healthy volunteers.
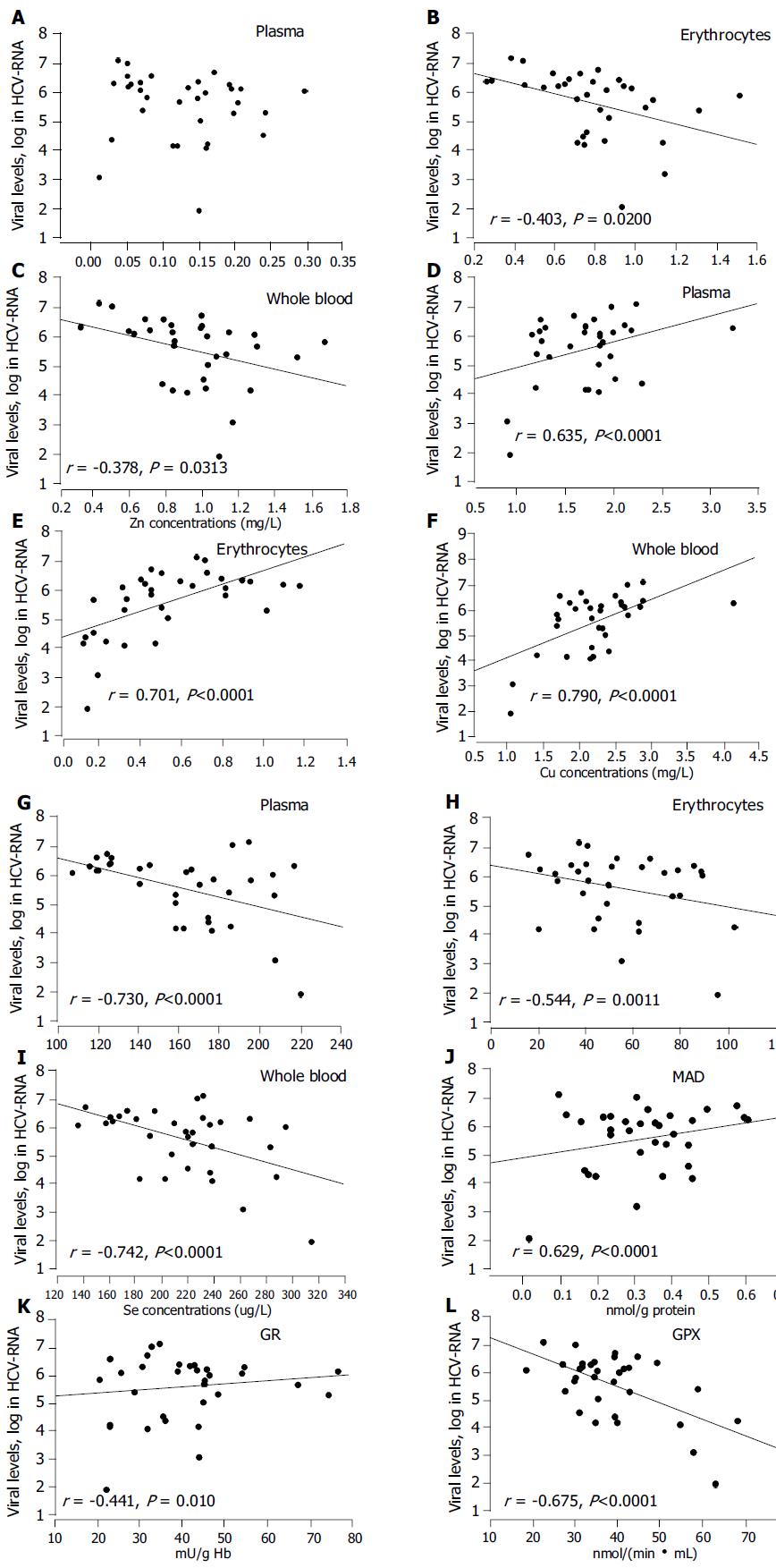
RESULTS: The contents of zinc (Zn), and selenium (Se) in plasma and erythrocytes were significantly lower in hepatitis C patients than in the controls. On the contrary, copper (Cu) levels were significantly higher. Furthermore, plasma and erythrocyte MDA levels, and the SOD and GR activities in erythrocytes significantly increased in hepatitis C patients compared to the controls. However, the plasma GPX activity in patients was markedly lower. Plasma Se (r = -0.730, P < 0.05), Cu (r = 0.635), and GPX (r = -0.675) demonstrated correlations with HCV-RNA loads. Significant correlation coefficients were also observed between HCV-RNA levels and erythrocyte Zn (r = -0.403), Se (r = -0.544), Cu (r = 0.701) and MDA (r = 0.629) and GR (r = 0.441).
CONCLUSION: The levels of Zn, Se, Cu, and oxidative stress (MDA), as well as related anti-oxidative enzymes (GR and GPX) in blood have important impact on the viral factors in chronic hepatitis C. The distribution of these parameters might be significant biomarkers for HCV.
- Citation: Ko WS, Guo CH, Yeh MS, Lin LY, Hsu GSW, Chen PC, Luo MC, Lin CY. Blood micronutrient, oxidative stress, and viral load in patients with chronic hepatitis C. World J Gastroenterol 2005; 11(30): 4697-4702
- URL: https://www.wjgnet.com/1007-9327/full/v11/i30/4697.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i30.4697
Essential micronutrients are involved in many metabolic pathways in the liver, such as enzymatic functions and protein synthesis, oxidative damage and anti-oxidant defense, immunological competence, interferon therapy response regulations, and alterations of the virus genomes[1-5]. Reactive oxygen species (ROS) have also been implicated in a number of hepatic pathologies in exacerbating liver diseases[6-10]. The oxidant production associated with immune reactions against viral hepatitis leads to the formation of hepatocellular carcinoma (HCC)[8]. Therefore, the changes in micronutrients and their demolishing effects against oxidative stress are factors for viral hepatitis pathogenesis.
HCV is a major cause of chronic liver disease. HCV infection frequently leads to chronic hepatitis with increasing risk of developing liver cirrhosis and HCC. Interferon with or without ribavirin is the only drug with proven efficacy in treating chronic HCV infections[11-13]. Unfortunately, these therapeutic models maintain the rate of sustained virologic response (SVR) to approximately 10-40%[14-16]. The effective advancement in the antiviral treatments against chronic hepatitis C is necessary.
There are several factors that attribute to the failure in achieving a SVR for the majority of patients[17-19]. Hepatic iron deposit has been identified as one of these factors[20-23]. Iron depletion and zinc supplementation[24,25] may improve the response of chronic hepatitis C patients to interferon treatment. Moreover, viral factors may affect the outcome of the therapy. Zhang et al.[26], indicated that selenium-dependent glutathione peroxidase (GPX) modules are encoded in the RNA viruses. The presence of zinc ion also decreases the HIV-1 reverse transcriptase activity[27]. It is conceivable that the micronutrient status may affect the HCV load and viral replication, leading to significant changes in reported SVR rates. However, there is limited information about the distribution of micronutrients and their effects on viral production.
Our present study aimed to examine the levels of micronutrients (zinc, copper, iron, selenium), and malondi-aldehyde (MDA) which is an indirect marker for oxidative stress in blood. Moreover, superoxide dismutase (SOD), glutathione reductase (GR) and GPX activities were assessed. In addition, the relationships among these parameters and HCV-RNA levels in patients with chronic hepatitis C were investigated.
This study contained 33 patients with chronic hepatitis C including 20 men and 13 women (from 2002 to 2003). The mean age of the patients was 49.5 ± 2.4 years. All patients underwent serological and biochemical analyses. Diagnosis of chronic hepatitis C was based on elevated serum alanine aminotransferase levels for at least 6 mo, and consistent detection of serum HCV-RNA. All patients were negative for hepatitis B surface antigen and HIV, and none had liver cirrhosis or renal disease.
A control group of 31 healthy volunteers (17 men and 14 women) was recruited from blood donors aged 43.2 ± 1.7 years. They underwent a routine medical examination prior to the blood collection.
Venous blood samples were collected from patients prior to treatment. Erythrocytes were pelleted by centrifugation and washed thrice with cold isotonic saline. The plasma and erythrocyte zinc (Zn), copper (Cu), and iron (Fe) levels were determined by flame atomic absorption spectrophotometry (932 plus, GBC, Australia) as previously described[28]. The accessory hydride formation system (HG3000), also from GBC, was used for determining selenium (Se) concentrations. All samples were analyzed in triplicate. Serum “second-generation” reference materials (SeronormTM Trace Elements Serum) were purchased from Nycomed, Oslo, Norway.
Thiobarbituric acid substances reacted with products of lipid peroxidation, mainly MDA, producing a pink color compound that could be measured at 535 nm. Following the protocol in Richard et al.[29], thiobarbituric acid levels were determined in plasma and erythrocytes of patients and controls. Results were expressed as nanomoles of MDA per milliliter in plasma and as nanomoles of MDA per gram protein in erythrocytes. Protein concentration was determined using the Coomassie protein assay (Pierce, Rockford, IL, USA) with bovine serum albumin as the standard.
Erythrocyte SOD and GPX activities in plasma were determined with RANSOD kits (Randox, San Diego) and Cayman GPx assay kits (Cayman Chemical, USA) respectively. All values were expressed as units per gram hemoglobin or units per milliliter. One unit of SOD was defined as the amount of enzyme necessary to produce 50% inhibition in the p-iodonitrotetrazolium reduction rate. The activity of GR in erythrocytes was also measured at 340 nm using the commercial kits (GR340, OxisResearch). One GR activity unit was defined as the amount of enzyme catalyzing the reduction of 1 mmoL of GSSG.
Additionally, viral RNA was detected and quantitative HCV-RNA was determined by Amplicor (Roche Molecular Diagnostics) and expressed as the log of copies of RNA per milliliter. RNA was extracted from serum samples following the manufacturer’s instructions (QIAamp viral RNA kit from Qiagen Inc.). This assay had a lower limit of 100 copies/mL.
Data were expressed as mean±SE. Significant differences in variables between two groups were tested by Student’s t-test. P < 0.05 was considered statistically significant. Linear regressions were used to analyze the correlation among variables.
Table 1 shows the values of specific micronutrients in plasma of chronic hepatitis C patients and healthy subjects. There was a significant decrease in Zn and Se and a statistical increase in Cu concentrations of the patients (P < 0.05). However, plasma Fe levels revealed no significant difference (P > 0.05).
The erythrocyte concentration of Cu in the patients was significantly higher than that in the healthy controls, and the Fe concentrations were not significantly different between two groups. Furthermore, Se and Zn levels were significantly lower in erythrocytes of the patients (Table 2).
Table 3 summarizes the values of parameters related to oxidative stress. Erythrocyte and plasma MDA increased significantly in patients. Also, the erythrocyte Cu, Zn-SOD, and GR activities increased significantly in the patients compared to the controls. In contrast, the plasma Se-dependent GPX activity in hepatitis C patients was markedly lower.
Figures 1A-C shows that there was a significant negative relationship between Zn contents in erythrocytes/whole blood and HCV-RNA levels (by log) in patients. In plasma, however, no significant correlation was observed. A positive correlation was noted between plasma Cu and HCV-RNA levels in patients, and an even stronger correlation between the Cu in erythrocytes/whole blood and HCV-RNA levels (Figures 1D-F).
The Se levels in plasma/erythrocytes/whole blood had a statistically significant negative correlation with HCV-RNA levels, whereas a better correlation was found in plasma or whole blood (Figures 1G-I, r = -0.730, -0.742, P < 0.0001).
No significant correlation was found between MDA in plasma and HCV-RNA levels (P > 0.05, data not presented). However, erythrocyte MDA production was positively correlated with HCV-RNA concentration (Figure 1J, r = 0.629, P < 0.0001). Additionally, HCV-RNA levels correlated with both erythrocyte GR (Figure 1K, r = 0.441, P = 0.01) and plasma GPX (Figure 1L, r = -0.675, P < 0.0001) activities. The activities of SOD did not correlate with the HCV-RNA levels (P > 0.05, data not shown).
The purpose of our study was to find the levels of blood micronutrient and oxidative stress in patients with chronic hepatitis C, and to search for linkages between the HCV-RNA levels and micronutrient status, as well as between oxidative stress and the presence of antioxidant enzymes.
The essential micronutrients (Zn, Cu, Fe, and Se) might exacerbate liver disease in case of deficiency, imbalance, or toxicity[5,30]. They are also linked to the process of oxidation during chronic liver damage[6,7,31]. In the present investigation, the levels of Zn and Se in plasma and erythrocytes of HCV-infected patients decreased significantly compared to healthy subjects. On the contrary, the Cu levels in patients were significantly higher than those in the control group. Nevertheless, alterations of these micronutrients in plasma and erythrocytes varied in different magnitudes.
Decreasing levels of Zn, Se, or increasing Cu levels were also noted in sera of hepatitis cases[31-33]. HCV- and HIV-co-infected patients showed markedly lower blood Se levels compared to HIV-infected patients without concomitant HCV infection[34]. However, no significant difference in blood Zn and Se concentrations was observed between chronic hepatitis C patients and controls[35,36]. One possible explanation for this discrepancy is that, the subjects in the above two studies could not be distinguished by the type of hepatitis, and patients with hepatitis A-D may have different blood concentrations of Zn and Se. The present study did not find significantly higher values of erythrocyte Fe in the patients to coincide with the results in Loguercio et al.[36], whereas some data in the literature show an obvious increase of Fe contents both in the liver and in the serum of these patients[37,38]. Thus, the pretreatment levels of blood Fe might not be a proper marker for the iron status in patients with chronic hepatitis C infection.
Although the precise causes remain to be elucidated, there is evidence that cytokines might alter the levels of serum trace elements in viral hepatitis[32]. It was reported that inflammatory cytokines are higher in HCV-infected individuals than in normal individuals[39,40]. Increased Cu levels might result from inflammatory responses[32]. Therefore, the present results suggest that changes of Zn, Cu, and Se levels in plasma and erythrocytes of patients with chronic hepatitis C are directly related to the pathology developed in the liver.
ROS plays a crucial role in the induction and progression of liver disease, and are involved in the transcription and activation of a large series of cytokines that could induce production of ROS. Some studies indicate that treatment of high serum Se and Zn levels leads to reduction of inflammatory reaction in hepatitis patients[41,42]. In addition to the anti-inflammatory reaction, Se or Zn has antioxidant and immuno-modulatory effects[43-45]. Copper is also associated with the inflammatory response and oxidative stress[46,47]. In the present study, decreased activity of Se-dependent GPX and increased Cu, Zn-SOD, and GR activities either in plasma or in erythrocytes suggest that anti-oxidative capability is limited during circulation. The presence of significant increase in MDA levels also indicates a possible oxidative insult in these patients. Liver cirrhosis induces a significant decrease in Se and Zn levels, another indication of presence of oxidative stress[36]. The levels of MDA have been correlated with the severity of chronic hepatitis[45]. There is evidence that the production of free radicals increases while anti-oxidant defense decreases significantly in all types of liver damage[6-9]. Therefore, supportive nutrients, Zn, Cu, and Se, and oxidative stress might be sensitive indicators for the degree of liver injuries and the sustained response to therapy in chronic hepatitis C patients.
The outcome of HCV infection is also thought to depend on the balance between the rate of viral replication, rapidity, and specificity and the effectiveness of the host immune response. Few studies have focused on the relationship between circulating pool of these immune-regulated micronutrients and HCV-RNA contents. Our present results suggest that there is a markedly negative relationship between HCV-RNA titer (in log) and either erythrocyte or whole blood Zn concentrations. Similarly, the inverse correlations between HCV-RNA and blood Se levels observed in this study suggest that significant higher viral loads are correlated with lower blood Zn and Se levels in HCV-infected patients.
In addition, higher levels of blood Cu are markedly correlated with higher HCV-RNA concentrations. Little is known about the possible regulatory mechanism of micronutrients in the pathogenesis of HCV infection. However, these nutrients are known to assist in immune-mediated response and involve in the alteration of virus genomes[5,27,45,48]. It is suggested that the distribution of Zn, Se, and Cu might affect sustained response to therapy in patients with chronic hepatitis C. Therefore, these micronutrients may be involved in multiple points in the immune pathogenesis of HCV infection that is essential for viral clearance.
The oxidative stress is high in hepatitis patients, and there are significant correlations among HCV-RNA and erythrocyte MDA, erythrocyte GR and plasma GPX activities. Zhang et al.[26], reported that Se-dependent GPX modules are encoded in a number of RNA viruses, including HIV-1, HCV, Coxsackie’s virus B3, and HIV-2 virus. A HCV-encoded GPX gene might demonstrate that oxidant stress is associated with HCV disease progression. In HIV-infected patients, the decline in Se levels is greater than that in those with HCV co-infection[34]. Se deficiency increases the virulence of CVB3 infection, which is encoded by the GPX gene within Keshan disease’s cofactor[26,49]. It has been proposed that Se-dependent GPX participates directly in immune cytotoxicity, enabling neutrophils and macrophages to complete intracellular lysis of phagocytosed cells. In previous investigations, an inverse relationship between Se level and HBV infection incidence was found[50]. Zn has also been studied for its antiviral effect against HIV, rhinovirus, and herpes virus[24]. Moreover, Zn supplementation enhances the response to interferon therapy in chronic hepatitis C patients[25]. Zn is necessary for the dimerization of interferon, which activates the interferon receptor[51]. It is apparent that erythrocyte and plasma Zn, Se, and Cu levels and oxidative stress are associated with HCV-RNA levels.
In conclusion, the distribution of Zn, Cu, Se levels and MDA product, GPX and GR activities in blood may be an additional host-specific parameter (outside of predictive viral factors) with a predictive value for the responsiveness of patients to interferon/ribavirin therapy. Furthermore, these results may be affected by immunocytokines as a host-defense system during HCV infection.
Great appreciation is extended to Miss Anna Hsu of Oregon State University for the English editing of the manuscript.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
Co-first-authors: Wang-Sheng Ko and Chih-Hung Guo
Co-correspondents: Wang-Sheng Ko
| 1. | Beisel WR. Single nutrients and immunity. Am J Clin Nutr. 1982;35:417-468. [PubMed] |
| 2. | Evans GW. Zinc and its deficiency diseases. Clin Physiol Biochem. 1986;4:94-98. [PubMed] |
| 3. | Bray TM, Bettger WJ. The physiological role of zinc as an antioxidant. Free Radic Biol Med. 1990;8:281-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 567] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 4. | Bhaskaram P. Micronutrient malnutrition, infection, and immunity: an overview. Nutr Rev. 2002;60:S40-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Ozcelik D, Ozaras R, Gurel Z, Uzun H, Aydin S. Copper-mediated oxidative stress in rat liver. Biol Trace Elem Res. 2003;96:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1-85. [PubMed] |
| 7. | Peterhans E. Reactive oxygen species and nitric oxide in viral diseases. Biol Trace Elem Res. 1997;56:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Jain SK, Pemberton PW, Smith A, McMahon RF, Burrows PC, Aboutwerat A, Warnes TW. Oxidative stress in chronic hepatitis C: not just a feature of late stage disease. J Hepatol. 2002;36:805-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 136] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Loguercio C, Federico A. Oxidative stress in viral and alcoholic hepatitis. Free Radic Biol Med. 2003;34:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 328] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 10. | Toubi E, Kessel A, Goldstein L, Slobodin G, Sabo E, Shmuel Z, Zuckerman E. Enhanced peripheral T-cell apoptosis in chronic hepatitis C virus infection: association with liver disease severity. J Hepatol. 2001;35:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | National Institutes of Health Consensus Development Conference Panel statement: management of hepatitis C. Hepatology. 1997;26:2S-10S. [PubMed] |
| 12. | McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2509] [Cited by in RCA: 2434] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 13. | Poynard T, Leroy V, Cohard M, Thevenot T, Mathurin P, Opolon P, Zarski JP. Meta-analysis of interferon randomized trials in the treatment of viral hepatitis C: effects of dose and duration. Hepatology. 1996;24:778-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 388] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 14. | Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet. 1998;352:1426-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1667] [Cited by in RCA: 1640] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 15. | Thévenot T, Regimbeau C, Ratziu V, Leroy V, Opolon P, Poynard T. Meta-analysis of interferon randomized trials in the treatment of viral hepatitis C in naive patients: 1999 update. J Viral Hepat. 2001;8:48-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Hoofnagle JH. Management of hepatitis C: current and future perspectives. J Hepatol. 1999;31 Suppl 1:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Neuman MG, Benhamou JP, Malkiewicz IM, Akremi R, Shear NH, Asselah T, Ibrahim A, Boyer N, Martinot-Peignoux M, Jacobson-Brown P. Cytokines as predictors for sustained response and as markers for immunomodulation in patients with chronic hepatitis C. Clin Biochem. 2001;34:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Ho SB, Nguyen H, Tetrick LL, Opitz GA, Basara ML, Dieperink E. Influence of psychiatric diagnoses on interferon-alpha treatment for chronic hepatitis C in a veteran population. Am J Gastroenterol. 2001;96:157-164. [PubMed] |
| 19. | Kumar D, Wallington-Beddoe C, George J, Lin R, Samarasinghe D, Liddle C, Farrell GC. Effectiveness of interferon alfa-2b/ribavirin combination therapy for chronic hepatitis C in a clinic setting. Med J Aust. 2003;178:267-271. [PubMed] |
| 20. | Van Thiel DH, Friedlander L, Fagiuoli S, Wright HI, Irish W, Gavaler JS. Response to interferon alpha therapy is influenced by the iron content of the liver. J Hepatol. 1994;20:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 154] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Olynyk JK, Reddy KR, Di Bisceglie AM, Jeffers LJ, Parker TI, Radick JL, Schiff ER, Bacon BR. Hepatic iron concentration as a predictor of response to interferon alfa therapy in chronic hepatitis C. Gastroenterology. 1995;108:1104-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 181] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Kageyama F, Kobayashi Y, Murohisa G, Shimizu E, Suzuki F, Kikuyama M, Souda K, Kawasaki T, Nakamura H. Failure to respond to interferon-alpha 2a therapy is associated with increased hepatic iron levels in patients with chronic hepatitis C. Biol Trace Elem Res. 1998;64:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Carlo C, Daniela P, Giancarlo C. Iron depletion and response to interferon in chronic hepatitis C. Hepatogastroenterology. 2003;50:1467-1471. [PubMed] |
| 24. | Nagamine T, Takagi H, Takayama H, Kojima A, Kakizaki S, Mori M, Nakajima K. Preliminary study of combination therapy with interferon-alpha and zinc in chronic hepatitis C patients with genotype 1b. Biol Trace Elem Res. 2000;75:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Takagi H, Nagamine T, Abe T, Takayama H, Sato K, Otsuka T, Kakizaki S, Hashimoto Y, Matsumoto T, Kojima A. Zinc supplementation enhances the response to interferon therapy in patients with chronic hepatitis C. J Viral Hepat. 2001;8:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Zhang W, Ramanathan CS, Nadimpalli RG, Bhat AA, Cox AG, Taylor EW. Selenium-dependent glutathione peroxidase modules encoded by RNA viruses. Biol Trace Elem Res. 1999;70:97-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Sabbioni E, Blanch N, Baricevic K, Serra MA. Effects of trace metal compounds on HIV-1 reverse transcriptase: an in vitro study. Biol Trace Elem Res. 1999;68:107-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Guo CH, Huang CJ, Chiou YL, Hsu GS. Alteration of trace element distribution and testis ACE activity in mice with high peritoneal aluminum. Biol Trace Elem Res. 2002;86:145-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Richard MJ, Portal B, Meo J, Coudray C, Hadjian A, Favier A. Malondialdehyde kit evaluated for determining plasma and lipoprotein fractions that react with thiobarbituric acid. Clin Chem. 1992;38:704-709. [PubMed] |
| 30. | Buck WB, Ewan RC. Toxicology and adverse effects of mineral imbalance. Clin Toxicol. 1973;6:459-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Pramoolsinsap C, Promvanit N, Komindr S, Lerdverasirikul P, Srianujata S. Serum trace metals in chronic viral hepatitis and hepatocellular carcinoma in Thailand. J Gastroenterol. 1994;29:610-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Kalkan A, Bulut V, Avci S, Celik I, Bingol NK. Trace elements in viral hepatitis. J Trace Elem Med Biol. 2002;16:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Czuczejko J, Zachara BA, Staubach-Topczewska E, Halota W, Kedziora J. Selenium, glutathione and glutathione peroxidases in blood of patients with chronic liver diseases. Acta Biochim Pol. 2003;50:1147-1154. [PubMed] |
| 34. | Look MP, Rockstroh JK, Rao GS, Kreuzer KA, Barton S, Lemoch H, Sudhop T, Hoch J, Stockinger K, Spengler U. Serum selenium, plasma glutathione (GSH) and erythrocyte glutathione peroxidase (GSH-Px)-levels in asymptomatic versus symptomatic human immunodeficiency virus-1 (HIV-1)-infection. Eur J Clin Nutr. 1997;51:266-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 90] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Loguercio C, De Girolamo V, Federico A, Feng SL, Cataldi V, Del Vecchio Blanco C, Gialanella G. Trace elements and chronic liver diseases. J Trace Elem Med Biol. 1997;11:158-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Loguercio C, De Girolamo V, Federico A, Feng SL, Crafa E, Cataldi V, Gialanella G, Moro R, Del Vecchio Blanco C. Relationship of blood trace elements to liver damage, nutritional status, and oxidative stress in chronic nonalcoholic liver disease. Biol Trace Elem Res. 2001;81:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Sikorska K, Stalke P, Lakomy EA, Michalska Z, Witczak-Malinowska K, Stolarczyk J. Disturbances of iron metabolism in chronic liver diseases. Med Sci Monit. 2003;9 Suppl 3:64-67. [PubMed] |
| 38. | Metwally MA, Zein CO, Zein NN. Clinical significance of hepatic iron deposition and serum iron values in patients with chronic hepatitis C infection. Am J Gastroenterol. 2004;99:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Malaguarnera M, Di Fazio I, Romeo MA, Restuccia S, Laurino A, Trovato BA. Elevation of interleukin 6 levels in patients with chronic hepatitis due to hepatitis C virus. J Gastroenterol. 1997;32:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Neuman MG, Benhamou JP, Martinot M, Boyer N, Shear NH, Malkiewicz I, Katz GG, Suneja A, Singh S, Marcellin P. Predictors of sustained response to alpha interferon therapy in chronic hepatitis C. Clin Biochem. 1999;32:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Sartori M, Andorno S, Rigamonti C, Boldorini R. Chronic hepatitis C treated with phlebotomy alone: biochemical and histological outcome. Dig Liver Dis. 2001;33:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Selimoglu MA, Aydogdu S, Unal F, Yüce G, Yagci RV. Serum zinc status in chronic hepatitis B and its relationship to liver histology and treatment results. Pediatr Int. 2001;43:396-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Tănăsescu C, Băldescu R, Chirulescu Z. Interdependence between Zn and Cu serum concentrations and serum immunoglobulins (IgA, IgM, IgG) in liver diseases. Rom J Intern Med. 1996;34:217-224. [PubMed] |
| 44. | Takagi H, Nagamine T, Abe T, Takayama H, Sato K, Otsuka T, Kakizaki S, Hashimoto Y, Matsumoto T, Kojima A. Zinc supplementation enhances the response to interferon therapy in patients with chronic hepatitis C. J Viral Hepat. 2001;8:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Cunningham-Rundles S, Ahrn S, Abuav-Nussbaum R, Dnistrian A. Development of immunocompetence: role of micronutrients and microorganisms. Nutr Rev. 2002;60:S68-S72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Fisher AE, Naughton DP. Vitamin C contributes to inflammation via radical generating mechanisms: a cautionary note. Med Hypotheses. 2003;61:657-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Klein D, Lichtmannegger J, Finckh M, Summer KH. Gene expression in the liver of Long-Evans cinnamon rats during the development of hepatitis. Arch Toxicol. 2003;77:568-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Taylor EW, Nadimpalli RG, Ramanathan CS. Genomic structures of viral agents in relation to the biosynthesis of selenoproteins. Biol Trace Elem Res. 1997;56:63-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Beck MA, Shi Q, Morris VC, Levander OA. Rapid genomic evolution of a non-virulent coxsackievirus B3 in selenium-deficient mice results in selection of identical virulent isolates. Nat Med. 1995;1:433-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 244] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 50. | Yu SY, Zhu YJ, Li WG. Protective role of selenium against hepatitis B virus and primary liver cancer in Qidong. Biol Trace Elem Res. 1997;56:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 207] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 51. | Radhakrishnan R, Walter LJ, Hruza A, Reichert P, Trotta PP, Nagabhushan TL, Walter MR. Zinc mediated dimer of human interferon-alpha 2b revealed by X-ray crystallography. Structure. 1996;4:1453-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 175] [Article Influence: 6.0] [Reference Citation Analysis (0)] |