Published online Aug 14, 2005. doi: 10.3748/wjg.v11.i30.4638
Revised: November 1, 2004
Accepted: November 4, 2004
Published online: August 14, 2005
AIM: To investigate the association of cyclooxygenase-2 (COX-2) expression with angiogenesis and the number and type of inflammatory cells (macrophages/Kupffer cells; mast cells) within primary hepatocellular carcinoma (HCC) tissues and adjacent non-tumorous (NT) tissues.
METHODS: Immunohistochemistry for COX-2, CD34, CD68 and mast cell tryptase (MCT) was performed on 14 well-characterized series of liver-cirrhosis-associated HCC patients. COX-2 expression and the number of inflammatory cells in tumor lesions and surrounding liver tissues of each specimen were compared. Moreover, COX-2, CD34 staining and the number of inflammatory cells in areas with different histological degrees within each tumor sample were comparatively analyzed.
RESULTS: The percentage of COX-2 positive cells was significantly higher in NT tissues than in tumors. COX-2 expression was higher in well-differentiated HCC than in poorly-differentiated tissues. Few mast cells were observed within the tumor mass, whereas a higher number was observed in the surrounding tissue, especially in peri-portal spaces of NT tissues. Abundant macrophages/ Kupffer cells were observed in NT tissues, whereas the number of cells was significantly lower in the tumor mass. However, a higher cell number was observed in the well-differentiated tumor and progressively decreased in relation to the differentiation grade. Within the tumor, a positive correlation was found between COX-2 expression and the number of macrophages/Kupffer cells and mast cells. Moreover, there was a positive correlation between CD34 and COX-2 expression in tumor tissues. Comparison between well- and poorly-differentiated HCC showed that the number of CD34-positive cells decreased with dedifferentiation. However, COX-2 was the only independent variable showing a positive correlation with CD34 in a multivariate analysis.
CONCLUSION: The presence of inflammatory cells and COX-2 expression in liver tumor suggests a possible relationship with tumor angiogenesis. COX-2 expressing cells and the number of macrophages/Kupffer cells and mast cells decrease with progression of the disease.
- Citation: Cervello M, Foderà D, Florena AM, Soresi M, Tripodo C, D’Alessandro N, Montalto G. Correlation between expression of cyclooxygenase-2 and the presence of inflammatory cells in human primary hepatocellular carcinoma: Possible role in tumor promotion and angiogenesis. World J Gastroenterol 2005; 11(30): 4638-4643
- URL: https://www.wjgnet.com/1007-9327/full/v11/i30/4638.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i30.4638
Cyclooxygenase-2 (COX-2) is an inducible immediate early gene associated with inflammation, cell growth and differen-tiation, prevention of apoptosis and tumorigenesis[1]. A substantial body of evidence supports the role of COX-2 in the angiogenesis of a variety of human malignancies[2-5]. Recent studies have already shown an increased expression of COX-2 in patients with liver disease, suggesting the role of COX-2 in chronic liver disease and during the progression of HCC[6-8]. In addition, COX-2 expression is reported to correlate with tumor angiogenesis in patients with hepatitis C or B virus-associated HCC[9,10].
Macrophages are an important source of angiogenic activity in wound healing, cancer, and chronic inflammation. They can produce various growth factors and cytokines that promote angiogenesis. The presence of infiltrating macrophages is closely associated with angiogenesis in several types of malignancies, including melanoma[11], breast[12], prostate[13] and lung[14] cancer, glioma[15], cervical[16] and esophageal carcinoma[17]. In the liver, infiltrating macrophages and Kupffer cells which are considered as resident macrophages, play an essential role not only in host defense but also in homeostatic responses of tissue[18-20]. However, their role in the process of tumor progression and angiogenesis is not well understood.
Mast cells (MCs) circulate in blood as progenitors and undergo terminal differentiation into mature cells only when they enter the tissues. Mast cells release a variety of factors known to enhance angiogenesis, namely heparin, histamine and tryptase, as well as cytokines, such as transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α), interleukin-8 (IL-8), fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor (VEGF). MC density is highly correlated with the extent of both normal and pathological angiogenesis in chronic inflammatory diseases and tumors[21,22]. MCs are present in both normal and pathological livers[23,24]. Their role in tumor angiogenesis is not entirely clear, although MCs are of primary importance in the transition from sinusoidal to capillary-type endothelial cells during HCC growth.
Although, as quoted above, some studies on the liver have evaluated the relationship between COX-2 and angiogenesis, or the relationship between the presence of macrophages and mast cells and the different liver pathologies, no studies are available to date on the possible relationship between the various parameters. Therefore, we investigated the association of COX-2 expression with the number of microvessels, the number and type of inflammatory cells in primary hepatocellular carcinoma and adjacent non-tumorous tissues; compared within the same tumor specimen of the two areas with the greatest difference in differentiation grade, and evaluated which of the parameters analyzed could play a prominent role in the neoangiogenesis of HCC.
The study included 14 primary HCC patients whose main clinical characteristics are shown in Table 1. Diagnosis was made according to the pathological findings in all cases. All the patients with known cirrhosis were enrolled in a prospective study for HCC screening. The disease was associated with the presence of serum HCV antibodies in all cases. None of the patients was positive for HBsAg. HCC was histologically graded by two pathologists (AMF and CT) and divided into well-differentiated (WD), moderately-differentiated (MD) or poorly-differentiated (PD) types. Nine of the fourteen patients showed different histological grades (WD+MD+PD) in a single nodule (Table 1). However, a total of 23 tumor sites (11 well-differentiated and 12 poorly-differentiated) were analyzed (Table 1). In order to analyze the different parameters during tumor progression and to avoid variability between the different patients, we compared the two areas with the greatest difference in differentiation grade (i.e. well-differentiated vs poorly-differentiated, n = 9) within the same tumor specimen.
| n | Age (yr) | Sex | Child | ALT | AST | Tumor size (cm) | Histology pattern | Histology grading |
| 1 | 65 | F | A5 | 26 | 55 | 2.8 | PS | WD |
| 2 | 64 | M | A6 | 21 | 17 | 3 | TR + PS + COM | WD + MD + PD |
| 3 | 62 | M | A5 | 53 | 44 | 7 | TR | WD + MD + PD |
| 4 | 63 | M | B7 | 91 | 334 | 3 | TR | PD |
| 5 | 66 | M | B7 | 145 | 302 | 1 | TR + PS | WD |
| 6 | 77 | M | A5 | 32 | 24 | 3 | TR + PS | WD + MD + PD |
| 7 | 53 | F | A6 | 161 | 186 | 2 | TR + PS + COM | WD + MD + PD |
| 8 | 70 | F | A6 | 25 | 38 | 3 | TR + PS + COM | WD + MD + PD |
| 9 | 75 | F | B7 | 42 | 55 | 2 | TR | PD |
| 10 | 65 | M | A5 | 41 | 37 | 2.5 | TR + COM | WD + MD + PD |
| 11 | 56 | F | A5 | 28 | 57 | 3.5 | TR + PS + COM | WD + MD + PD |
| 12 | 77 | M | A6 | 44 | 47 | 2.7-2.2 | TR + PS + COM | PD |
| 13 | 61 | M | A6 | 21 | 17 | 2.5-4.5 | TR + PS + COM | WD + MD + PD |
| 14 | 78 | M | A5 | 210 | 388 | 6 | TR + PS | WD + MD + PD |
Specimens were fixed in formalin and embedded in paraffin. Four micrometer-thick sections were cut, dewaxed and hydrated. In the case of COX-2 and mast cell tryptase (MCT) staining, the sections were first heated in a microwave oven (3-4 cycles of 5 min each) in 10 mmol/L citrate buffer (pH 6.0) and then washed twice with PBS for 5 min. All sections were incubated with 30 mL/L hydrogen peroxide in methanol for 5 min to inhibit endogenous peroxidase. Immunohistochemistry was performed by the streptavidin-biotin complex (StreptABC) using the following antibodies: rabbit polyclonal antibody against COX-2 (Cayman, Chemical, MI, USA) at a dilution of 1:100 for 2 h at 37°C, and mouse mAb against CD68 (clone PG-M1, Dako, Copenhagen, Denmark) at a dilution of 1:50 and CD34 (Clone Qbend/10, Menarini, Florence, Italy) at a dilution of 1:30, or anti-human mast cell tryptase (clone AA1, Dako, Copenhagen, Denmark) at a dilution of 1:150 for 30 min at room temperature. Sections were then incubated for 30 min at room temperature with biotinylated anti-rabbit or anti-mouse immunoglobulin diluted in PBS and streptavidin-biotin complex for 30 min at room temperature. The color was developed with 3-amino-9-ethyl-carbazole (AEC) (Dako, Copenhagen, Denmark) for 5-10 min at room temperature and counterstained with Mayer hematoxylin for 3 min.
Immunohistochemical staining for COX-2 was semi-quanti-tatively evaluated by two independent observers (AMF, CT) using a scale of 0-5, according to both degree and intensity of staining, in which 0: negative, 1: positive staining in 1-20% of cells, 2: in 21-40%, 3: in 41-60%, 4: in 61-80% and 5 ≥ 81%.
When the number of macrophages and mast cells was determined, the CD68-positive and MCT-positive cells were counted respectively. Intra-tumoral microvessels were assessed by immunostaining with anti-CD34. All stained endothelial cells or cell clusters were counted as one micro-vessel. Branching structures were counted as a single vessel. The same two evaluators (AMF and CT) performed the counts. Briefly, stained sections were observed at 100× magnification to identify the areas with the highest number of positive cells. Counts were performed in five regions at 200×magnification. A scale of 0-5 was used for CD68, in which 0: negative, 1: 1-15 positive cells, 2: 16-30, 3: 31-45, 4: 45-60 and 5 ≥ 61. In the case of CD34, a scale of 0-4 was used, in which 0: negative, 1: 1-20 microvessels, 2: 21-40, 3: 41-60, 4: ≥ 61. For MCT, the mean of the five counts was used directly because a small number of cells were observed.
Data were expressed as median and range (min-max). The Mann-Whitney U test and Spearman’s rank correlation test were used when appropriate. Multiple linear regression analysis was used to study the association between increased values of CD34 and values of COX-2, CD68, and MCT. The linear regression equation was used to describe a linear relationship between the dependent variable (CD34) and one or more explanatory variables. P < 0.05 was considered statistically significant.
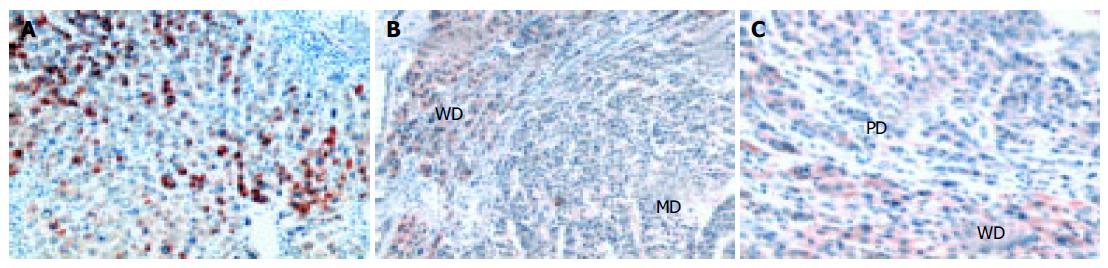
COX-2 expression was detected in all the HCCs studied by immunohistochemical analysis. The percentage of COX-2 positive cells was significantly higher in non-tumor (NT) tissues than in tumors (P < 0.0001) (Figure 1 and Table 2). COX-2 showed a diffuse cytoplasmic localization in NT hepatocytes, whereas it showed a cytoplasmic dot-like pattern in tumor cells. COX-2 expression tended to be higher in well-differentiated than in poorly-differentiated HCC tissues (z = 4.2, P < 0.0001). As shown in Figures 1B and C, a clear difference in COX-2 staining was observed at the boundary of HCC tissues with different histological grades.
| LC | HCC | z | P | |
| COX-2 | 5 | 1 | 3.7 | < 0.0001 |
| (3 - 5) | (0 - 5) | |||
| CD68 | 5 | 3 | 4.2 | < 0.0001 |
| (3 - 5) | (0 - 4) | |||
| MCT | 4 | 1 | 4 | < 0.0001 |
| (2 - 18) | (0 - 10) |
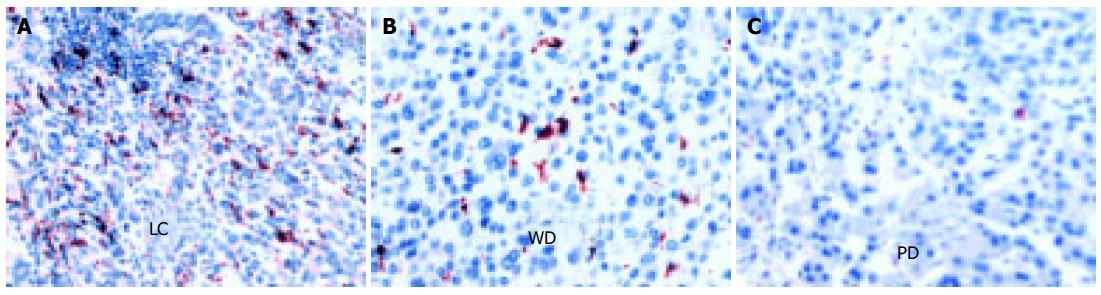
In this study, anti-CD68, an anti-human macrophage antibody, was used to identify the macrophages. However, in the CD68-positive (CD68+) cells, short spindle cells were considered to be Kupffer cells, whereas migrating macrophages were those with oval shape and abundant cytoplasm. The total number of CD68+cells was calculated in all cases. The expression of CD68+cells was significantly higher in non-tumor tissue than in tumor itself (P < 0.0001, Figure 2 and Table 2). In addition, the number of CD68+cells reduced as the histological grade decreased (Figures 2B and C) and was completely absent in some cases of poorly-differentiated HCC.
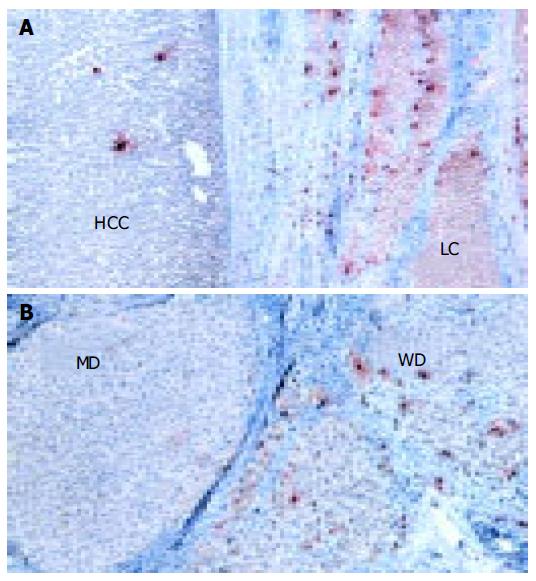
Mast cells were recognized by using anti-human tryptase mAb. Tryptase is a neutral protease contained in the secretory granules of MCs. Tryptase-positive mast cells (MCT) were abundant and mainly localized in the portal tracts of non-tumorous liver tissues (Figure 3A). The number of MCs was significantly lower in HCC tissue than in surrounding cirrhotic liver tissue (P < 0.0001, Figure 3A and Table 2). However, different histological grades of HCC showed different number of MCs (Figure 3B), well-differentiated HCC showed the highest number of MCs which tended to decrease in less-differentiated HCC (z = 4.1, P < 0.0001).
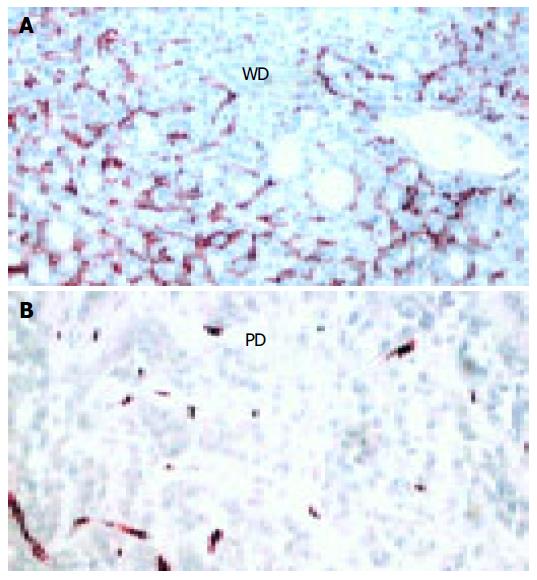
The presence of microvessels inside the tumor mass was evaluated by staining endothelial cells with anti-CD34 antibody. In the surrounding cirrhotic tissue CD34-positive cells were found only in the portal tracts and septa. As shown in Figure 4, CD34+cells were clearly found in well-differentiated HCC, whereas poorly-differentiated HCC showed few or no vessels (Figure 4B). The mean score was 2 (0-14) in well- and 0 (0-2) in poorly- differentiated HCC (z = 3.3, P < 0.001).
COX-2 expression as evaluated in all HCC sites (WD-HCC+PD-HCC, n = 23) significantly correlated with the presence of macrophages (P < 0.001), mast cells (P < 0.0001) and CD34 expression (P < 0.0001). The presence of CD68+ cells significantly correlated with the number of MCT (P < 0.0001) and CD34 expression (P < 0.001). The number of MCT in HCC significantly correlated with CD34 expression (P < 0.001).
To confirm the relationship between angiogenesis expr-essed as CD34 (dependent variable) and other parameters (independent variables), multiple linear regression analysis was performed. A close association was found between CD34 and COX-2 (β = 0.69, P < 0.002, R2 = 0.65), while no association was found between CD68 (P < 0.07) and MCT (P = not significant).
Tumor angiogenesis is essential for tumor growth, and depends on angiogenic factors produced by tumor cells and/or infiltrating cells in tumor tissue. The tumor microenvironment largely orchestrated by inflammatory cells is an indispensable participant factor in the neoplastic process. The inflammatory component of a developing neoplasm includes a varied leukocyte population, e.g., macrophages, neutrophils, eosinophils, and mast cells, all of which are variably loaded
with an assorted array of cytokines, cytotoxic mediators including reactive oxygen species, serine-, cysteine-, and metalloproteases, membrane-perforating agents, and soluble cell killing mediators, such as TNF-α, interleukines, and interferons[24,25].
In recent years, a number of studies have correlated COX-2 expression with tumor angiogenesis[2-5]. In addition, accumulation of inflammatory cells, macrophage and mast cells coupled with angiogenesis can be found in the literature[11-17,20,21].
In this study, we evaluated the association of COX-2 expression with angiogenesis and the number and type of inflammatory cells in primary HCC and surrounding NT tissues, as well as their association with differentiation grades in HCC tissues. A previous study demonstrated that dediff-erentiation occurs with time and tumor growth[26]. In our study, a single tumor node showing areas with different histological grades was found in most HCC tissues. This allowed us to compare well-differentiated tumors with poorly-differentiated tumors in the same subject, and therefore to determine the role of molecules and cells analyzed during HCC progression.
We found that COX-2 expression and the number of macrophages/Kupffer cells and MCs were higher in adjacent NT tissues than in tumors. However, due to our limited patient population (n = 14) no correlation was observed by comparing each parameter in NT and tumor tissues in the same patient (data not shown). In addition, no correlation was found between the presence of inflammatory cells and COX-2 expression in peri-tumorous cirrhotic liver (data not shown).
We found that COX-2 was expressed in all HCC cases, which is in agreement with the results reported by Koga[6] and Bae[8]. COX-2 expression was higher in well-differentiated HCC than in poorly-differentiated HCC, suggesting that COX-2 may be involved in the early stages of hepatocarcinogenesis. The reduction of COX-2 expression during tumor progression is not common in all types of cancer. A possible explanation of this different behavior is that, in some cell types COX-2 over-expression may cause a growth disadvantage during tumor progression. This is in agreement with the results of Trifan et al[27], who reported that COX-2 overexpression is able to induce cell cycle arrest in a variety of cell types.
Although the functions of intra-tumor macrophages/ Kupffer cells and MCs are still unclear, two hypotheses have been advanced about their possible role in tumor lesions. One hypothesis suggests that both cell types are important in the host defense mechanism and anti-tumor effects, the other postulates that they may directly promote tumor growth, invasion and neovascularity. Our results, in agreement with previous observations, show that there is a significant difference in the number of CD68+cells between well- and poorly-differentiated HCC, and that the latter contains few of these cells. Similarly, the number of MCT decreases significantly with tumor dedifferentiation. These results suggest that the decrease in inflammatory cells is closely related to the differentiation grade and tumor progression. In view of these results, it is reasonable to hypothesize that both cell types may have an inhibitory effect on HCC cell proliferation.
Moreover, the data of this study seem to suggest a corre-lation between both cell types and COX-2 expression and tumor angiogenesis, although COX-2 expression is the only independent variable that shows a significant positive correlation with CD34.
In conclusion, there is a relationship between COX-2 expression and the neovasculature of human HCC. Therefore, it is likely that COX-2 inhibition may block HCC-associated angiogenesis, thus providing a rationale for the use of selective COX-inhibitors in the treatment of this malignancy.
We thank Dr. Lydia Giannitrapani, Nadia Lampiasi and Professor Vito Franco for their valuable discussion.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Cao Y, Prescott SM. Many actions of cyclooxygenase-2 in cellular dynamics and in cancer. J Cell Physiol. 2002;190:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 339] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 2. | Joo YE, Rew JS, Seo YH, Choi SK, Kim YJ, Park CS, Kim SJ. Cyclooxygenase-2 overexpression correlates with vascular endothelial growth factor expression and tumor angiogenesis in gastric cancer. J Clin Gastroenterol. 2003;37:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Chapple KS, Scott N, Guillou PJ, Coletta PL, Hull MA. Interstitial cell cyclooxygenase-2 expression is associated with increased angiogenesis in human sporadic colorectal adenomas. J Pathol. 2002;198:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Davies G, Salter J, Hills M, Martin LA, Sacks N, Dowsett M. Correlation between cyclooxygenase-2 expression and angiogenesis in human breast cancer. Clin Cancer Res. 2003;9:2651-2656. [PubMed] |
| 5. | Chu J, Lloyd FL, Trifan OC, Knapp B, Rizzo MT. Potential involvement of the cyclooxygenase-2 pathway in the regulation of tumor-associated angiogenesis and growth in pancreatic cancer. Mol Cancer Ther. 2003;2:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 204] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Koga H, Sakisaka S, Ohishi M, Kawaguchi T, Taniguchi E, Sasatomi K, Harada M, Kusaba T, Tanaka M, Kimura R. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology. 1999;29:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 303] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 7. | Kondo M, Yamamoto H, Nagano H, Okami J, Ito Y, Shimizu J, Eguchi H, Miyamoto A, Dono K, Umeshita K. Increased expression of COX-2 in nontumor liver tissue is associated with shorter disease-free survival in patients with hepatocellular carcinoma. Clin Cancer Res. 1999;5:4005-4012. [PubMed] |
| 8. | Bae SH, Jung ES, Park YM, Kim BS, Kim BK, Kim DG, Ryu WS. Expression of cyclooxygenase-2 (COX-2) in hepatocellular carcinoma and growth inhibition of hepatoma cell lines by a COX-2 inhibitor, NS-398. Clin Cancer Res. 2001;7:1410-1418. [PubMed] |
| 9. | Rahman MA, Dhar DK, Yamaguchi E, Maruyama S, Sato T, Hayashi H, Ono T, Yamanoi A, Kohno H, Nagasue N. Coexpression of inducible nitric oxide synthase and COX-2 in hepatocellular carcinoma and surrounding liver: possible involvement of COX-2 in the angiogenesis of hepatitis C virus-positive cases. Clin Cancer Res. 2001;7:1325-1332. [PubMed] |
| 10. | Cheng AS, Chan HL, To KF, Leung WK, Chan KK, Liew CT, Sung JJ. Cyclooxygenase-2 pathway correlates with vascular endothelial growth factor expression and tumor angiogenesis in hepatitis B virus-associated hepatocellular carcinoma. Int J Oncol. 2004;24:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Shimizu T, Abe R, Nakamura H, Ohkawara A, Suzuki M, Nishihira J. High expression of macrophage migration inhibitory factor in human melanoma cells and its role in tumor cell growth and angiogenesis. Biochem Biophys Res Commun. 1999;264:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 156] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625-4629. [PubMed] |
| 13. | Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A. Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol. 2000;17:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 157] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Takanami I, Takeuchi K, Kodaira S. Tumor-associated macrophage infiltration in pulmonary adenocarcinoma: association with angiogenesis and poor prognosis. Oncology. 1999;57:138-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 120] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Nishie A, Ono M, Shono T, Fukushi J, Otsubo M, Onoue H, Ito Y, Inamura T, Ikezaki K, Fukui M. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res. 1999;5:1107-1113. [PubMed] |
| 16. | Davidson B, Goldberg I, Gotlieb WH, Lerner-Geva L, Ben-Baruch G, Agulansky L, Novikov I, Kopolovic J. Macrophage infiltration and angiogenesis in cervical squamous cell carcinoma--clinicopathologic correlation. Acta Obstet Gynecol Scand. 1999;78:240-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Ohta M, Kitadai Y, Tanaka S, Yoshihara M, Yasui W, Mukaida N, Haruma K, Chayama K. Monocyte chemoattractant protein-1 expression correlates with macrophage infiltration and tumor vascularity in human esophageal squamous cell carcinomas. Int J Cancer. 2002;102:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Tanaka M, Nakashima O, Wada Y, Kage M, Kojiro M. Pathomorphological study of Kupffer cells in hepatocellular carcinoma and hyperplastic nodular lesions in the liver. Hepatology. 1996;24:807-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 95] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Bortolami M, Venturi C, Giacomelli L, Scalerta R, Bacchetti S, Marino F, Floreani A, Lise M, Naccarato R, Farinati F. Cytokine, infiltrating macrophage and T cell-mediated response to development of primary and secondary human liver cancer. Dig Liver Dis. 2002;34:794-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Ribatti D, Vacca A, Nico B, Crivellato E, Roncali L, Dammacco F. The role of mast cells in tumour angiogenesis. Br J Haematol. 2001;115:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Norrby K. Mast cells and angiogenesis. APMIS. 2002;110:355-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 266] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 22. | Hagmann W, Hacker HJ, Buchholz U. Resident mast cells are the main initiators of anaphylactic leukotriene production in the liver. Hepatology. 1992;16:1477-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Terada T, Matsunaga Y. Increased mast cells in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Hepatol. 2000;33:961-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med. 2000;248:171-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 509] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 25. | Wahl LM, Kleinman HK. Tumor-associated macrophages as targets for cancer therapy. J Natl Cancer Inst. 1998;90:1583-1584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Sugihara S, Nakashima O, Kojiro M, Majima Y, Tanaka M, Tanikawa K. The morphologic transition in hepatocellular carcinoma. A comparison of the individual histologic features disclosed by ultrasound-guided fine-needle biopsy with those of autopsy. Cancer. 1992;70:1488-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Trifan OC, Smith RM, Thompson BD, Hla T. Overexpression of cyclooxygenase-2 induces cell cycle arrest. Evidence for a prostaglandin-independent mechanism. J Biol Chem. 1999;274:34141-34147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |