Published online Jan 21, 2005. doi: 10.3748/wjg.v11.i3.372
Revised: November 16, 2003
Accepted: February 12, 2004
Published online: January 21, 2005
AIM: To investigate whether gastric myoelectrical activity was impaired in patients with chronic pancreatitis (CP) and to explore the role of pancreatic enzyme in regulating gastric myoelectrical activity.
METHODS: Twenty CP patients and 20 controls participated in the study. Gastric myoelectrical activity was recorded by a homemade electrogastrography (EGG) device. Two experiments were carried out. In experiment one, EGG was recorded in both controls and CP patients. While in experiment two, either pancreatic enzymes or placebo was given together with test meals. Spectral analysis was used to generate various EGG parameters.
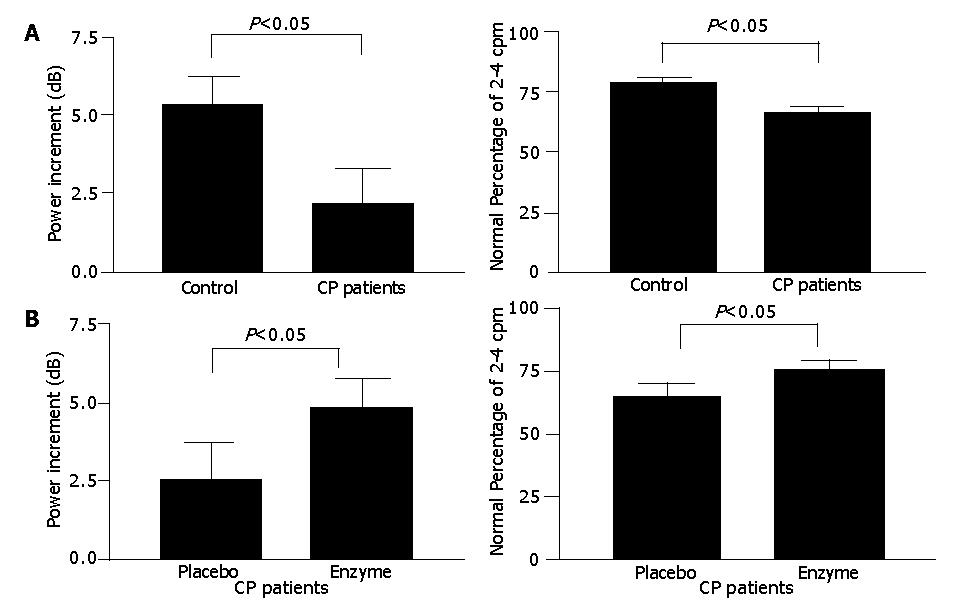
RESULTS: The control subjects, but not the CP patients, showed typically increased postprandial dominant frequency. The postprandial dominant power (DP) increment (2.24±1.13 vs 5.35±0.96 dB, P = 0.04) and the percentage of normal 2-4 cpm slow waves (63.0±3.8% vs 77.4 ±3.1%, P<0.05) were lower in CP patients when compared with the control. In the 20 CP patients, the DP increment (4.76±1.02 vs 2.53±1.20 dB, P<0.05) and the postprandial percentage of normal 2-4 cpm (74.4±2.8% vs 64.8 ±5.7%, P<0.05) were significantly higher with pancreatic enzyme replacement than the placebo.
CONCLUSION: CP patients have an abnormal postprandial stomach myoelectricity showing poor response in dominant frequency/power and regularity, whereas these abnormalities are corrected after pancreatic enzyme replacement. Maldigestion is likely to be the factor leading to abnormal postprandial gastric myoelectricity of CP patients.
- Citation: Lu CL, Chen CY, Luo JC, Chang FY, Lee SD, Wu HC, Chen J. Impaired gastric myoelectricity in patients with chronic pancreatitis: Role of maldigestion. World J Gastroenterol 2005; 11(3): 372-376
- URL: https://www.wjgnet.com/1007-9327/full/v11/i3/372.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i3.372
Gastrointestinal (GI) motility and pancreatic exocrine secretion are mutually regulated in concert. For example, the cyclical nature of interdigestive pancreatic secretion was synchronized with the periodic activity of migratory motor complex (MMC)[1,2]. Chronic pancreatitis (CP) offers an ideal model to study whether severely damaged pancreatic parenchyma in turn leads to disturbed gastric or intestinal motor function. To the authors’ knowledge, a few studies have been conducted to address this probable influence, with controversial results regarding the GI motility in CP patients. For instance, MMC of the stomach or the small intestine remained unchanged either in a canine model of total pancreatomy or in CP patients[3,4]. On the other hand, gastric emptying was accelerated in CP patients[5-7]. Interestingly, pancreatic enzyme replacement was found to restore this enhanced gastric emptying in CP patients[6]. However, other investigators found that the fractional gastric emptying rate (volume emptied into duodenum divided by gastric volume) remained eventually unchanged after the correction for gastric emptying[8].
Stomach myoelectricity or slow wave, which is omnipresent with a regular rhythm of about 3 cycles/min (cpm), can be recorded by abdominal surface electrodes, a technique called electrogastrography (EGG). Abnormal gastric rhythm recorded from EGG was suggestive of an impaired gastric pacemaker setting which usually determines the timing and maximum rate of gastric contractions[9-11]. EGG itself is a non-invasive and simple measure for the assessment of stomach myoelectrical rhythm in physiological or pathological situation. It has been shown that abnormal stomach rhythm exists in early pregnancy with nausea, motion sickness, diabetic gastroparesis, etc[12-14]. Even patients with stomach cancer manifested abnormal rhythm of slow waves[15]. While there is a mutual relationship between GI motility and pancreatic exocrine function, it is unknown whether CP patients have stomach dysrhythmia. Thus, the aim of the present study was to evaluate the gastric myoelectricity in CP patients and to determine whether pancreatic enzyme replacement could restore some EGG parameters if abnormal gastric myoelectricity did exist.
This protocol was approved by the Institutional Ethics Committee. After given informed written consent, 20 healthy volunteers (16M/4F, 56.8±17.6 years, range: 30-80) and 20 consecutive CP patients (17M/3F, 57.4±14.5 years, range: 28-84) were invited to participate in the study. None of the control subjects had any history of either acute or chronic pancreatitis. Nor did they have any dyspeptic symptoms. Similar to previous reports, the diagnosis of CP was established by a typical clinical history and imaging characteristic abnormalities on ultrasonography, computed tomography, and endoscopic retrograde cholangiopancreaticography in all patients[3,7]. CP history could be traced back to 1-10 years ago. Etiological factors for CP included alcohol consumption in 18 patients and idiopathic in 2. Four patients had obvious diabetes and were well controlled by an oral hypoglycemic agent. None of the enrolled CP patients had any evidence of autonomic neuropathy or other major extrapancreatic alcoholic or diabetic complications. The exclusion criteria for this study were shown in the followings: pregnancy, concurrent malignancy, motion sickness, anorexia nervosa, diabetes of non-CP etiology, connective tissue diseases, gastric/duodenal ulcer and its complications, and history of chronic renal disease or surgical operations on the upper gastrointestinal tract.
The objective scale of dyspeptic symptoms of all studied subjects was assessed according to a standard protocol. Dyspeptic symptoms in CP patients were questioned at the time when he/she had quitted from pancreatic enzyme treatment for at least one week. The dyspeptic symptoms, including nausea, vomiting, anorexia, epigastric pain, epigastric fullness and early satiety, were questioned and scaled according to the following severity scale: grade 0 = not present; 1 = mild, occasional, slightly affecting daily activities; 2 = moderate, often affecting daily activities; 3 = severe, very often, with strong impact on concentration and daily activities[9].
EGG recording was performed in all subjects in the morning at 9:00 after an overnight fast. All the drugs that could influence the gastrointestinal motility, like prokinetics, anticholinergics, calcium channel blockers, were discontinued at least one day before the trial. Two experiments were carried out. In experiment 1, EGG was recorded in both control and CP patients. The CP patients were asked to stop pancreatic enzyme supplements at least 3 d before the EGG study. In experiment 2, the CP patients underwent two EGG recording sessions on two separate days in a random order, one session with placebo and the other with pancreatic enzyme supplement (Panzynorm® 2 tablets, each tablet containing amylase 9000 units, proteases 500 units, lipase, Nordmark Arzneimittel GmbH & Co. KG, Uretersen, Germany). Replacements were given to the CP patients together with the test meal.
During EGG recording, the subject was in a supine position and asked not to move, fall asleep or talk throughout the entire measurement. Abdominal skin was cleaned and gently scrubbed until redness appeared to reduce the skin-electrode impedance. Four pairs of silver-silver chloride electrodes filled with electrode jelly (Red Dot-2237, 3M, St. Paul, MN, USA) were placed on the examined area as previously described[9] . After 30 min of basal fasting recording, the studied individual was asked to consume a standard test meal, which included 250-mL milk and a piece of cake (347 kcal, carbohydrate 51 g, protein 13.2 g, and fat 10 g). A 30-min postprandial recording was then resumed. All EGG recordings were coded, and the investigator who performed quantitative analysis was blinded to the nature of EGG recording.
Our homemade EGG system included a signal acquisition device, a notebook PC (Pentium 166 MHz), and a power supply and electrodes[9]. Recorded signals were initially pre-amplified with an instrumental amplifier. A 4th order active high-pass filter with a cut-off frequency of 0.01 Hz and another 4th order active low-pass filter with a cut-off frequency of 0.5 Hz were installed to filter out unnecessary noises, such as the heartbeat. A 2.0 Hz precision clock source was designed to provide a stable 1 Hz sampling rate. An AT89C52 (Atmel, San Jose, CA, USA) microprocessor chip was installed, and interrupted by the precision clock. The microprocessor obtained recorded signals via a 12-bit analog/digital converter card, while the analog multiplexer processed the EGG signals. Finally, the digitized signals were transmitted to the PC via a RS-232 interface for on-line operation and further analysis. The EGG data stored on the PC were analyzed by using custom-made software as described in our previous publication[9,10]. The following EGG parameters were then obtained and calculated.
The frequency at which the overall power spectrum of an entire EGG recording displayed a peak power in the range of 1.0-9.0 cpm, was defined as the EGG dominant frequency (DF)[12] . The DF of EGG was shown to be equal to the frequency of the gastric slow wave measured from implanted serosal electrodes[16]. It was computed by using the smoothed power spectral analysis method[17]. Smoothed power spectral analysis was used to produce the overall power spectrum of EGG during each recording period, which was 30 min in the fasting state and 30 min after the test meal. The power at the DF in EGG power spectrum was defined as the EGG dominant power (DP)[12]. Previous studies showed that the relative change in EGG dominant power reflected gastric contractility[16-18]. The decibel (dB) unit was used to represent the power of EGG. With an assumption that a sinusoidal signal had an amplitude of A, power P in dB was expressed as P(dB) = 10×log10 (A2)[12].
The percent of regular 2-4 cpm gastric slow wave, which reflected the regularity of gastric myoelectrical activity, was defined as the percentage of time during which normal 2-4 cpm slow waves were present over the entire observation period. This was computed by using the adaptive running spectral analysis method[19]. Each EGG recording was divided into blocks of 1 min without overlap. The power spectrum of each 1-min EGG was calculated and examined to see if the peak power was within the range of 2-4 cpm. The 1-min EGG was defined as normal if the DP was within the 2-4 cpm range. Otherwise, it was defined as dysrhythmia.
All data were presented as mean±SE. Student’s t test was used for statistical analysis wherever it was considered appropriate. A P value <0.05 was considered statistically significant.
In our study, mild dyspepsia was encountered in the CP patients, whereas no dyspeptic symptoms were noted in the healthy controls (Total symptom score in CP patients vs healthy controls: 3.25±0.37 vs 0, P<0.05).
In experiment 1, no significant difference was found in the fasting EGG parameters between controls and CP patients. For example, the DF of EGG in the fasting state was similar between controls and CP patients (3.01±0.06 vs 3.03±0.07 cpm, P>0.05). Regarding slow wave power, the fasting DPs were also not different between controls and CP patients (38.5±0.8 vs 41.4±1.3 dB, P>0.05). For the regularity of gastric slow waves in the fasting state, there was also no difference in the percentage of normal rhythm (2-4 cpm) between CP patients and controls (63.9±5.0 vs 70.0±3.1%, P>0.05).
In contrast to the fasting state, some differences did exist in the postprandial EGG parameters between controls and CP patients (Figure 1A). Postprandially, the controls (3.22±0.10 vs 3.01±0.06 cpm, P<0.05) but not the CP patients (3.09±0.07 vs 3.03±0.07 cpm, P>0.05), manifested a typical increase in DF. The postprandial increase in DP was observed both in controls (43.8±1.3 vs 38.5±0.8 dB, P<0.05) and in CP patients (43.7±1.2 vs 41.4±1.3 dB, P<0.05). However, the increment of DP was markedly smaller in CP patients compared to controls (2.24±1.13 vs 5.35 ±0.96 dB, P = 0.04). The postprandial percentage of normal 2-4 cpm rhythm in CP patients was significantly lower when compared to controls (63.0±3.8 vs 77.4±3.1 %, P<0.05). The percentage of bradygastria (<2 cpm) in CP patients recorded in the postprandial state was much higher than that in controls (22.4±3.4 vs12.8±3.1%, P<0.05).
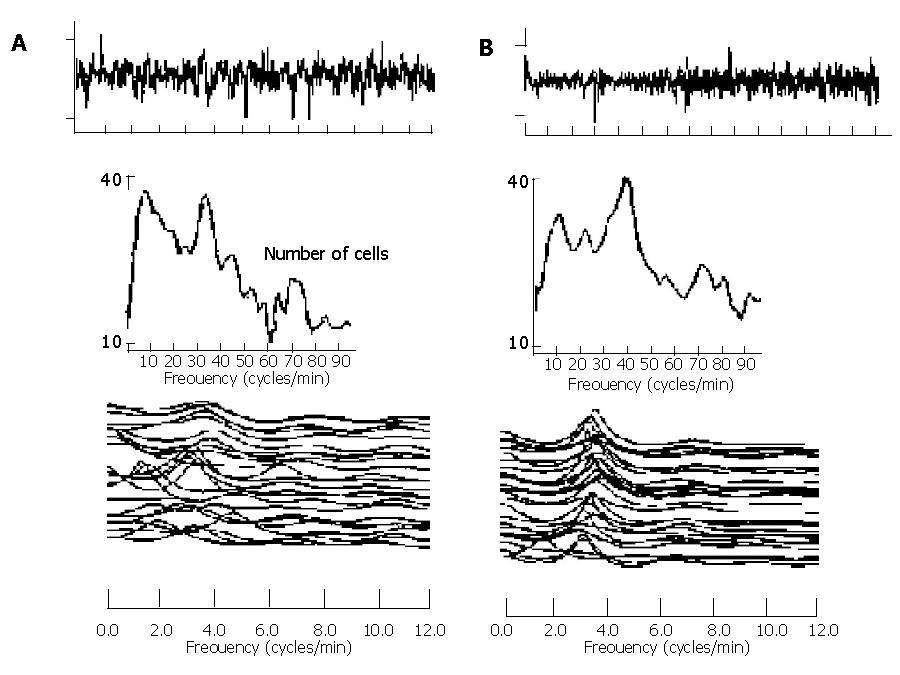
In experiment 2, we demonstrated that supplement of pancreatic enzyme could reverse the impaired EGG parameters toward normal (Figure 1B). The postprandial increase in the DF of EGG was observed in CP patients receiving pancreatic enzyme replacement (3.25±0.07 vs 3.08±0.07 cpm, P<0.05), but not in placebo group (3.05±0.08 vs 3.10±0.06 cpm, P<0.05). The postprandial increment of DP was also significantly higher (4.76±1.02 vs 2.53±1.20 dB, P<0.05) in pancreatic enzyme replacement group than in placebo group. The postprandial value of normal percentage of 2-4 cpm in CP patients significantly increased (64.8±5.7 vs 74.4 ±2.8 %, P<0.05) in the group with pancreatic enzyme supplement. The EGG response in a typical CP patient receiving either placebo or pancreatic enzyme supplement is shown in Figure 2.
To the best of our knowledge, there are no similar studies in the literature addressing the impairment of gastric myoelectricity in CP patients. Our study indicates that CP patients had impaired postprandial slow waves or impaired responses to the test meal and these impaired postprandial gastric myoelectrical parameters could be restored with replacement of pancreatic enzymes.
In the fasting state, exocrine pancreatic secretion fluctuated cyclically and closely coordinated with the gastrointestinal migrating motor complex[20,21]. It is debatable why pancreatic secretion should act in such a fluctuated manner. A few studies have been conducted to address this mystery. In a canine model of total pancreactomy, Malfertheiner et al[4] found no change in the normal interdigestive gastroduodenal motility pattern after surgical operation. Similarly, Pieramico et al[3] did not observe any changes in the interdigestive motility of CP patients when compared with controls. No study has previously been performed to evaluate the interdigestive and postprandial gastric myoelectrical activity in CP patients. We did not find any difference in recorded fasting DFs and DPs between CP patients and controls. Our observations may imply that the regularity and amplitude of the interdigestive stomach slow wave in CP patients remain intact despite pancreatic enzyme deficiency. Previous studies and our current data seem to suggest that pancreatic enzymes themselves do not have a major physiological role in the regulation of interdigestive or fasting gastric motility and myoelectrical activity.
It is interesting to know whether the postprandial motility in CP patients also remained unaltered. Gastric emptying was reported to be accelerated in patients with pancreatic insufficiency[6], but this acceleration was not confirmed in a subsequent study when gastric emptying rate was corrected to gastric volume output[8]. Furthermore, postprandial antral motility was reported unchanged[3]. In contrast, Vu et al[22] found that the duration of postprandial antroduodenal motor pattern was prolonged in CP patients, while the recorded antral motor index of CP patients was reduced in the first hour after meal ingestion. Impaired EGG parameters of DF, regularity of rhythm and DP were reported to be associated with poor stomach motility including gastric emptying and antral contractility[12]. Our data of impaired postprandial EGG parameters are partly in accordance with Vu’s observation of impaired postprandial antral motility.
It is unclear why CP patients had impaired postprandial stomach myoelectricity. It has been well established that the integrity of gastrointestinal motility depends on the final coordination between many neural, myogenic and hormonal factors[23,24]. Actually, CP patients with impaired exocrine function also had altered gut hormone release. For instance, the postprandial plasma level of cholecystokinin (CCK) was decreased in patients with pancreaticenzyme insufficiency[23]. Chen et al[25] indicated that intravenous infusion of CCK in the physiological dose decreased the postprandial EGG amplitude of normal humans, but did not change the frequency and regularity of stomach slow wave. Hence, it seems unlikely that impairment of the gastric myoelectrical activity we observed in CP patients could be attributed to CCK. On the other hand, the postprandial plasma level of a distal gut hormone-peptide YY (PYY) increased in CP patients[22]. This peptide is usually stored in the distal gut mucosa with the highest concentration and it behaves as one of the mediators functioning as the so called ileal-brake[26-28]. In fact, there are no available data regarding the impact of PYY on gastric myoelectricity. However, PYY infusion delayed gastric emptying and small intestinal transit in a dose-dependent manner in humans[28]. Interestingly, elevated postprandial level of PYY in CP patients was found to be able to return to normal after pancreatic enzyme supplement[22]. This observation was somewhat similar to our finding of restored abnormal postprandial EGG parameters after pancreatic enzyme supplement. It seems possible that the functioning PYY was one of the factors contributing to the abnormal postprandial stomach myoelectricity in CP patients.
Our study suggested that abnormal postprandial EGG parameters could be reversed to normal with pancreatic enzyme supplement. In addition, EGG parameters in CP patients could be impaired only in the postprandial state but not in the fasting state. All these observations suggest that the exocrine deficiency and subsequent maldigestion appeared responsible for the impaired gastric myoelectrical activity. Some previous studies also indicated that impaired GI motility could be restored after pancreatic enzyme supplement in CP patients[7,22]. Accordingly, it is likely that maldigestion might also act as one of the factors leading to abnormal antroduodenal motility in CP patients.
It may be argued that the disturbed gastric myoelectrical activity in CP patients observed in the present study might be attributed to diabetic autonomic neuropathy. Should this be the case, abnormalities would have been noted in both fasting and fed states as reported in a previous study, instead of abnormalities only in the fed state as shown in the current study. In addition, the blood glucose levels of the studied CP patients were well controlled, and there was no evidence of autonomic dysfunction in any of the enrolled CP patients. Finally, enzyme supplementation partially reversed the abnormal EGG parameters in CP patients. Consequently, we believe that the mechanism underlying impaired postprandial gastric myoelectrical activity may be the maldigestion rather than the pre-existing CP-elicited diabetes.
In conclusion, CP patients have normal fasting but abnormal postprandial gastric myoelectricity. The impairment involves DF and DP as well as percentage of normal gastric slow waves. These impairments can be restored with pancreatic enzyme replacement. Maldigestion is the likely factor leading to abnormal postprandial stomach myoelectricity in CP patients.
Assistant Editor Guo SY Edited by Qiu WS and Wang XL
| 1. | DiMagno EP, Hendricks JC, Go VL, Dozois RR. Relationships among canine fasting pancreatic and biliary secretions, pancreatic duct pressure, and duodenal phase III motor activity--Boldyreff revisited. Dig Dis Sci. 1979;24:689-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 76] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Layer P, Chan AT, Go VL, DiMagno EP. Human pancreatic secretion during phase II antral motility of the interdigestive cycle. Am J Physiol. 1988;254:G249-G253. [PubMed] |
| 3. | Pieramico O, Dominguez-Muñoz JE, Nelson DK, Böck W, Büchler M, Malfertheiner P. Interdigestive cycling in chronic pancreatitis: altered coordination among pancreatic secretion, motility, and hormones. Gastroenterology. 1995;109:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Malfertheiner P, Sarr MG, DiMagno EP. Role of the pancreas in the control of interdigestive gastrointestinal motility. Gastroenterology. 1989;96:200-205. [PubMed] |
| 5. | Brugge WR, Burke CA, Brand DL, Chey WY. Increased interdigestive pancreatic trypsin secretion in alcoholic pancreatic disease. Dig Dis Sci. 1985;30:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Long WB, Weiss JB. Rapid gastric emptying of fatty meals in pancreatic insufficiency. Gastroenterology. 1974;67:920-925. [PubMed] |
| 7. | Layer P, von der Ohe MR, Holst JJ, Jansen JB, Grandt D, Holtmann G, Goebell H. Altered postprandial motility in chronic pancreatitis: role of malabsorption. Gastroenterology. 1997;112:1624-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Regan PT, Malagelada JR, Dimagno EP, Go VL. Postprandial gastric function in pancreatic insufficiency. Gut. 1979;20:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Lu CL, Chen CY, Chang FY, Kang LJ, Lee SD, Wu HC, Kuo TS. Impaired postprandial gastric myoelectrical activity in Chinese patients with nonulcer dyspepsia. Dig Dis Sci. 2001;46:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Lu CL, Shidler N, Chen JD. Enhanced postprandial gastric myoelectrical activity after moderate-intensity exercise. Am J Gastroenterol. 2000;95:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Lu CL, Montgomery P, Zou X, Orr WC, Chen JD. Gastric myoelectrical activity in patients with cervical spinal cord injury. Am J Gastroenterol. 1998;93:2391-2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Chen JD, McCallum RW. Clinical applications of electrogastrography. Am J Gastroenterol. 1993;88:1324-1336. [PubMed] |
| 13. | Koch KL, Stern RM, Vasey M, Botti JJ, Creasy GW, Dwyer A. Gastric dysrhythmias and nausea of pregnancy. Dig Dis Sci. 1990;35:961-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 124] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Leahy A, Besherdas K, Clayman C, Mason I, Epstein O. Abnormalities of the electrogastrogram in functional gastrointestinal disorders. Am J Gastroenterol. 1999;94:1023-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Chang FY, Lu CL, Chen CY, Lee SD, Wu CW, Young ST, Wu HC, Kuo TS. Electrogastrographic characteristics in patients of stomach cancer. Dig Dis Sci. 2001;46:1458-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Chen JD, Schirmer BD, McCallum RW. Serosal and cutaneous recordings of gastric myoelectrical activity in patients with gastroparesis. Am J Physiol. 1994;266:G90-G98. [PubMed] |
| 17. | Abell TL, Malagelada JR. Glucagon-evoked gastric dysrhythmias in humans shown by an improved electrogastrographic technique. Gastroenterology. 1985;88:1932-1940. [PubMed] |
| 18. | Hamilton JW, Bellahsene BE, Reichelderfer M, Webster JG, Bass P. Human electrogastrograms. Comparison of surface and mucosal recordings. Dig Dis Sci. 1986;31:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 140] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Chen JD, Stewart WR, McCallum RW. Spectral analysis of episodic rhythmic variations in the cutaneous electrogastrogram. IEEE Trans Biomed Eng. 1993;40:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Johansson C, Schmidt DN, Hellström PM. Changed integrated gastrointestinal response to a mixed meal in exocrine pancreatic insufficiency. Pancreas. 1992;7:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Keane FB, DiMagno EP, Dozois RR, Go VL. Relationships among canine interdigestive exocrine pancreatic and biliary flow, duodenal motor activity, plasma pancreatic polypeptide, and motilin. Gastroenterology. 1980;78:310-316. [PubMed] |
| 22. | Vu MK, Vecht J, Eddes EH, Biemond I, Lamers CB, Masclee AA. Antroduodenal motility in chronic pancreatitis: are abnormalities related to exocrine insufficiency? Am J Physiol Gastrointest Liver Physiol. 2000;278:G458-G466. [PubMed] |
| 23. | Schmidt WE, Creutzfeldt W, Schleser A, Choudhury AR, Nustede R, Höcker M, Nitsche R, Sostmann H, Rovati LC, Fölsch UR. Role of CCK in regulation of pancreaticobiliary functions and GI motility in humans: effects of loxiglumide. Am J Physiol. 1991;260:G197-G206. [PubMed] |
| 24. | Thor P, Laskiewicz J, Konturek P, Konturek SJ. Cholecystokinin in the regulation of intestinal motility and pancreatic secretion in dogs. Am J Physiol. 1988;255:G498-G504. [PubMed] |
| 25. | Chen JD, Lin ZY, Parolisi S, McCallum RW. Inhibitory effects of cholecystokinin on postprandial gastric myoelectrical activity. Dig Dis Sci. 1995;40:2614-2622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89:1070-1077. [PubMed] |
| 27. | Pironi L, Stanghellini V, Miglioli M, Corinaldesi R, De Giorgio R, Ruggeri E, Tosetti C, Poggioli G, Morselli Labate AM, Monetti N. Fat-induced ileal brake in humans: a dose-dependent phenomenon correlated to the plasma levels of peptide YY. Gastroenterology. 1993;105:733-739. [PubMed] |
| 28. | Savage AP, Adrian TE, Carolan G, Chatterjee VK, Bloom SR. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut. 1987;28:166-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 240] [Article Influence: 6.3] [Reference Citation Analysis (0)] |